Figure 3.
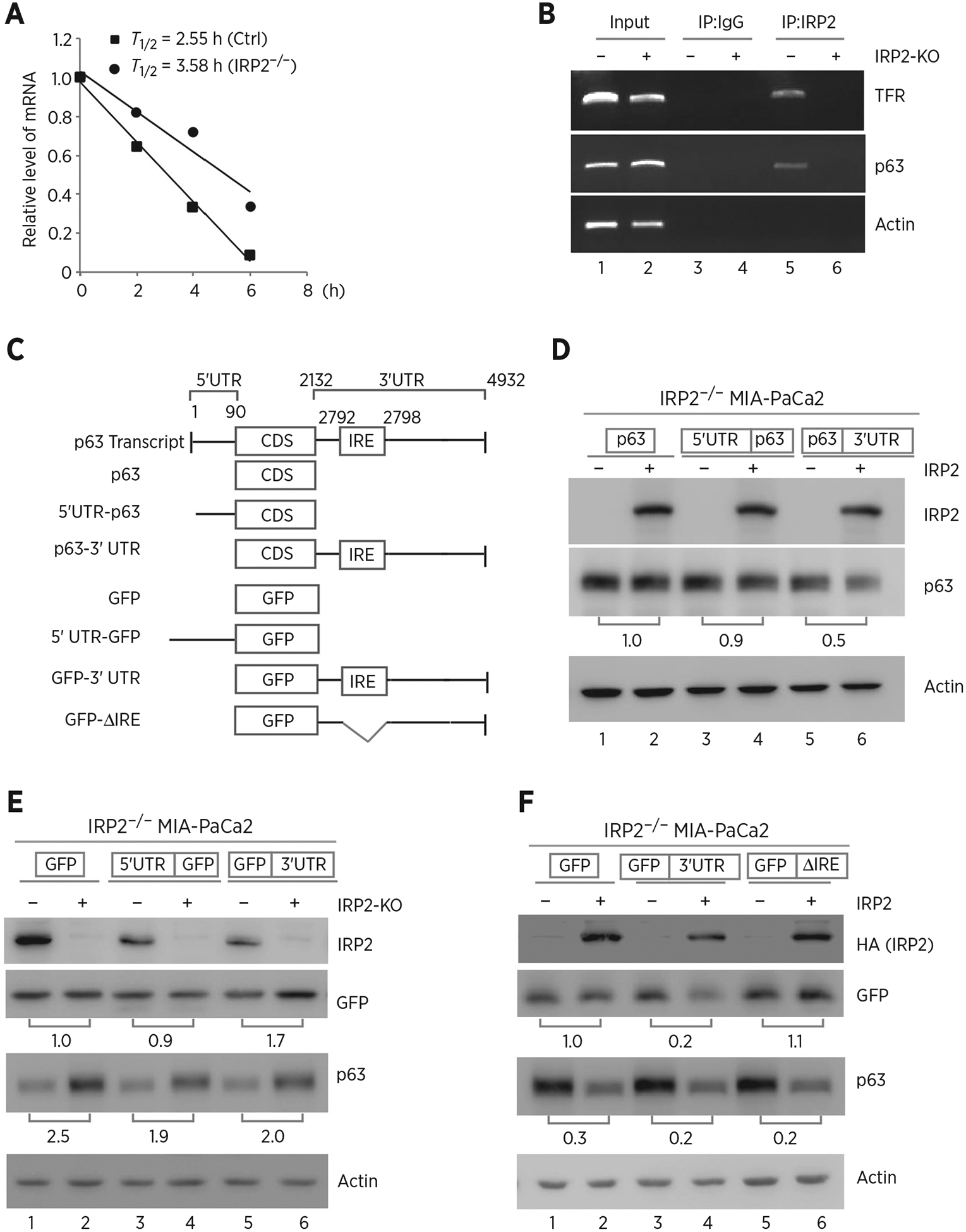
IRP2 regulates TP63 mRNA stability. A, The half-life of TP63 mRNA was determined in isogenic control or IRP2−/− MIA-PaCa2 cells. The level of TAp63 transcript was measured by qRT-PCR in isogenic control or IRP2−/− MIA-PaCa2 cells treated with 5,6- dichlorobenzimidazole-β-D-ribofuranoside (DRB; 100 μmol/L) for various times. mRNA was normalized by level of actin mRNA from triplicate samples and presented as mean ± SD. B, RNA-ChIP was performed with DNAase I–treated extracts from isogenic control (−) or IRP2−/− (+) MIA-PaCa2 cells with control IgG or anti-IRP2. Total RNAs were purified from immunocomplexes and subjected to RT-PCR to measure the levels of IRP2, p63, TFR and actin mRNAs. C, Schematic presentation of TAp63 transcript and TAp63 reporters that carry TAp63 coding region alone, or together with TAp63 5′ or 3′UTR. Also shown below is schematic presentation of GFP reporters that carry GFP coding region alone, or together with TAp63 5′UTR, 3′UTR, or a mutant 3′UTR with deletion of the putative IRE region. D, IRP2−/− MIA-PaCa2 cells were transfected with control pcDNA3 (−) or a vector expressing HA-IRP2 (+) along with a reporter that contains TAp63 coding region alone or together with TAp63 5′ or 3′UTR. Twenty-four hours posttransfection, cell lysates were collected and subjected to Western blot analysis to detect IRP2, TAp63 and actin. The level of proteins was normalized to that of actin, and the relative fold change is shown below each pair. E, Isogenic control (−) and IRP2-KO (+) MIA-PaCa2 cells were transfected with a reporter that contains GFP coding region alone or together with TAp63 5′ or 3′UTR. Twenty-four hours posttransfection, cell lysates were collected and subjected to Western blot analysis to detect IRP2, GFP, TAp63, and actin. The levels of GFP and TAp63 proteins were normalized to that of actin, and the relative fold change is shown below each pair. F, A putative IRE in TP63 3′UTR is recognized by and responsive to IRP2. IRP2−/− MIA-PaCa2 cells were transfected with control pcDNA3 (−) or a vector expressing HA-IRP2 (+) along with a GFP reporter as listed in C. Twenty-four hours posttransfection, cell lysates were collected and subjected to Western blot analysis to detect IRP2, GFP, TAp63, and actin proteins. The levels of GFP and TAp63 proteins were normalized to that of actin, and the relative fold change is shown below each pair.
