Abstract
MUTYH-associated polyposis (MAP) is a hereditary cancer syndrome that is caused by biallelic pathogenic variants in the MUTYH gene and should be evaluated for in patients with an attenuated colonic polyposis phenotype. Monoallelic pathogenic variants in MUTYH are associated with a moderate increased risk of colorectal cancer but not with the polyposis phenotype. We present a case of a patient presenting with multiple colonic adenomatous polyps whose germline testing revealed a heterozygous pathogenic variant in MUTYH in exon 13, c.1187G>A (p.Gly396Asp) as well as a heterozygous variant of unknown significance (VUS) in MUTYH in exon 14, c.1379T>C (p.Leu460Ser). We interpret the VUS as pathogenic in light of the patient’s phenotype; the fact that the VUS was in trans with a known pathogenic variant; and because all the in silico predictors suggested it was likely to be deleterious. This case highlights the importance of a gastroenterologist recognizing the indication for genetic testing in a patient with greater than ten adenomas, the importance of a genetic counselor in interpretation of results and is the first report of the specific variant in the literature with clinical information to suggest it is likely pathogenic.
Keywords: MUTYH, MYH-Associated polyposis, hereditary cancer syndrome, polyposis, Multiple Colorectal Adenomas
INTRODUCTION
MUTYH-associated polyposis (MAP) is an autosomal recessive hereditary cancer syndrome caused by biallelic pathogenic variants in the MUTYH gene that classically presents with an attenuated colonic polyposis phenotype and confers an approximately 80% lifetime risk of colorectal cancer (CRC) development [1, 2, 3]. MUTYH is a base excision repair gene and loss of activity leads to a high frequency of somatic alterations (guanine to thymine transversions) in the APC gene. Given the autosomal recessive inheritance, family history of CRC is often not present in contrast to Familial Adenomatous Polyposis syndrome (FAP) caused by pathogenic variants in APC.
Recent cross sectional studies [4, 5, 6] of highly selected patients with an attenuated phenotype with negative germline testing for pathogenic variants in the APC gene have reported a prevalence of MAP ranging from 5–14%, leading to an expert guideline recommendation [1] to test all patients with ten or more lifetime colonic adenomas for MAP and attenuated FAP. Recognition by the gastroenterologist of such patients is critical to insure referral for genetic counseling and testing. However, approximately 1–2% of the general population carries a single pathogenic variant in MUTYH and are, thus, monoallelic carriers. These patients do not have a polyposis phenotype, but may have a modestly increased lifetime risk of CRC ranging from 5–7% [3]. With the widespread clinical use of next generation sequencing (NGS) genetic panel testing, in which multiple pre-specified genes are sequenced in parallel on a single patient sample, both the spectrum of disease phenotypes is widening and the identification of unique pathogenic variants in a given gene is increasing [7]. It is thus critical to have access to genetic counselors when genetic testing is performed to help interpret the results and counsel patients appropriately.
CASE REPORT
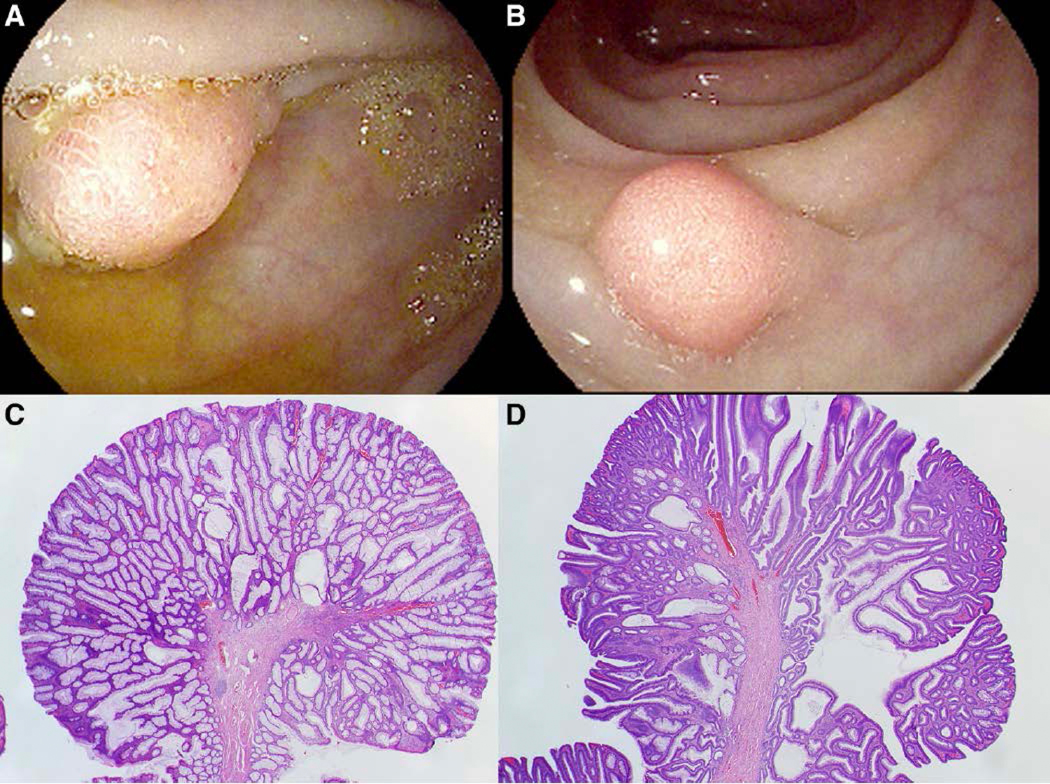
A 51 year-old man was referred to the Hereditary Gastrointestinal Cancer Prevention Program by his gastroenterologist for evaluation of a hereditary cancer syndrome after a colonoscopy performed for colon cancer screening revealed numerous (30–40) colonic polyps ranging in size from six millimeters to two centimeters (representative polyps shown in Figure 1), all of which were tubular and tubulovillous adenomas. The larger polyps were removed with endoscopic mucosal resection (EMR) and the smaller polyps were removed with standard cold snare polypectomy or hot snare polypectomy, as appropriate. A follow-up colonoscopy performed one year later revealed an additional fourteen polyps that were tubular adenomas, tubulovillous adenomas, and serrated adenomas. Colorectal cancer was not found on the colonoscopy, and the patient had no personal history of cancer. The patient had minimal contact with most of his family and was unaware of any family history of cancer. The patient was of Mexican descent.
Figure 1:
Endoscopic images of representative polyps are shown in panel A and B; histologic images with hematoxylin and eosin stain at 20X magnification showing a representative tubular adenoma (panel C) and tubulovillous adenoma (panel D).
The patient was referred for genetic counseling after his gastroenterologist recognized the presence of an attenuated colon polyposis phenotype despite no family history of cancers and age greater than 50. The differential diagnosis included attenuated FAP, MAP as well as several other hereditary cancer syndromes. An Invitae NGS panel (see Supplementary appendix for genes tested and report) returned with a heterozygous pathogenic variant in MUTYH in exon 13, c.1187G>A (p.Gly396Asp) as well as a heterozygous variant of unknown significance (VUS) in MUTYH in exon 14, c.1379T>C (p.Leu460Ser). In the case of the pathogenic variant c.1187G>A, it had previously been described in the literature [8] and was catalogued in ClinVar. The glycine residue which was replaced by aspartic acid is known to be highly conserved, and this missense change has been shown to affect MUTYH protein function [9], which all led to the variant being classified as pathogenic. The VUS in MUTYH in exon 14, c.1379T>C (p.Leu460Ser) had not been reported in the literature and was not present in population databases such as ClinVar and Leiden Open Variation Database (LOVD) and was thus reported as a VUS. However, c.1379T>C replaces highly conserved leucine with serine. There are large physiochemical differences between leucine and serine, and the in silico analysis predicted the missense change to affect the protein structure and function suggestive of a disruptive variant (see Supplementary appendix for details of in silico analysis). The location of the two variants within the MUTYH gene were close enough to allow the laboratory to determine that they are in trans based on the primary sequencing data.
Given the patient had a polyposis phenotype and monoallelic pathogenic variants in MUTYH are associated with increased risk for CRC but not polyposis, the VUS was interpreted clinically by the genetic counselor in conjunction with the gastroenterologist as likely pathogenic in light of the patient’s phenotype, the fact that the VUS was in trans with a known pathogenic variant and all the in silico predictors suggested it was likely to be deleterious. The patient was counseled to undergo surveillance according to guidelines for MAP caused by biallelic pathogenic variants in MUTYH rather than the less intensive surveillance recommended for patients with monoallelic pathogenic variants in MUTYH.
DISCUSSION
We present a case of a patient with a colonic polyposis phenotype with germline testing revealing a well-described monoallelic pathogenic variant in MUTYH and a separate VUS in trans in MUTYH; as a result of a multi-disciplinary approach to the patient’s care involving the gastroenterologist and genetic counselor, we interpreted the VUS to likely represent a newly reportable likely pathogenic variant.
MAP was first described in 2002 as an autosomal recessive polyposis syndrome associated with an increased risk of CRC development and has subsequently been found to account for 0.7% of all CRC cases and 2% of familial or early onset CRC [1]. Classically, patients present between the ages of 40–60 years with a variable number of adenomatous colorectal polyps, though generally less than 100 polyps, which helps differentiate it from FAP [2]. In terms of prognosis, patients have an 80% lifetime risk of CRC development and thus are recommended to undergo surveillance colonoscopy every one to two years as a means of prevention as well as early detection of CRC. Generally, the polyps can be managed endoscopically with polypectomy, including EMR for the larger polyps with the goal of debulking the polyp burden. Patients are referred for colectomy if the polyps become endoscopically unresectable or harbor advanced histologic features or frank malignancy.
There are a few alternative explanations in our particular case that were considered that deserve discussion. The first is that the monoallelic pathogenic variant alone caused the patient’s polyposis syndrome, the second is that a pathogenic variant in another untested gene is responsible for his polyposis or provided synergism with the pathogenic variant in MUTYH, and a third is that a cryptic variant in a gene that was tested for was not detected by current technology or that the variant occurred in a region not sequenced. In a cross-sectional study [10] of 90 patients with a clinical phenotype of attenuated familial adenomatous syndrome, four patients were found to have monoallelic pathogenic variants in MUTYH, though all of these patients had a markedly strong family history, which we did not identify in our patient. It seemed unlikely that the identified pathogenic variant alone caused the polyposis syndrome as this would imply a novel gain of function, which is unlikely given the variant has been described previously.
This case highlights several important concepts for the practicing gastroenterologist. The first is the importance of identification of patients who warrant further evaluation for a genetic cause of an attenuated polyposis syndromes. In this case, the recent guideline [1] recommending evaluation for genetic testing in any patient with greater than ten lifetime adenomas, irrespective of age, was recognized in this patient and was the basis for the referral for genetic counseling. Furthermore, this case highlights the importance of interpretation of genetic testing results in the context of the patient’s clinical phenotype. NGS panel testing is increasingly being used in clinical practice as it allows for simultaneous evaluation of multiple hereditary cancer syndromes. However, inconclusive results and VUS are common with NGS panel tests, with rates as high as 15–23% in colon panel tests [7]. These VUS often truly do not contribute to risk assessment, but can be re-classified as likely pathogenic or pathogenic as increasing data becomes available. As such, it is critical for the gastroenterologist to discuss the case with a genetic counselor to insure the genetic testing results are interpreted appropriately in the individual patient. In the case of our patient, we felt his phenotype combined with the details of the variant suggested that the VUS was likely to be clinically relevant and thus counseled the patient as if he had biallalic pathogenic variants in MUTYH causing MAP.
Supplementary Material
Acknowledgements
Funding/Grants: National Cancer Institute (K07CA212057) and American Gastroenterological Association grant both awarded to JKL
Footnotes
Informed consent to publish a case report: Obtained from patient
REFERENCES
- 1.Hegde M, Ferber M, Mao R et al. Acmg technical standards and guidelines for genetic testing for inherited colorectal cancer (lynch syndrome, familial adenomatous polyposis, and myh-associated polyposis). . [DOI] [PubMed] [Google Scholar]
- 2.Kastrinos F, Syngal S. Inherited colorectal cancer syndromes. Cancer journal. 2011;17:405–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodenberger M, Lindor NM. Lynch syndrome and myh-associated polyposis: Review and testing strategy. Journal of clinical gastroenterology. 2011;45:488–500 [DOI] [PubMed] [Google Scholar]
- 4.Sieber OM, Lipton L, Crabtree M et al. Multiple colorectal adenomas, classic adenomatous polyposis, and germ-line mutations in myh. The New England journal of medicine. 2003;348:791–799 [DOI] [PubMed] [Google Scholar]
- 5.Wang L, Baudhuin LM, Boardman LA et al. Myh mutations in patients with attenuated and classic polyposis and with young-onset colorectal cancer without polyps. Gastroenterology. 2004;127:9–16 [DOI] [PubMed] [Google Scholar]
- 6.Grover S, Kastrinos F, Steyerberg EW et al. Prevalence and phenotypes of apc and mutyh mutations in patients with multiple colorectal adenomas. JAMA. 2012;308:485–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LaDuca H, Stuenkel AJ, Dolinsky JS et al. Utilization of multigene panels in hereditary cancer predisposition testing: Analysis of more than 2,000 patients. Genetics in medicine. 2014;16:830–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nielsen M, Lynch H, Infante E, Brand R. Mutyh-associated polyposis In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A, eds. Genereviews((r)). Seattle (WA); 1993. [Google Scholar]
- 9.Ruggieri V, Pin E, Russo MT et al. Loss of mutyh function in human cells leads to accumulation of oxidative damage and genetic instability. Oncogene. 2013;32:4500–4508 [DOI] [PubMed] [Google Scholar]
- 10.Morak M, Laner A, Bacher U et al. Mutyh-associated polyposis - variability of the clinical phenotype in patients with biallelic and monoallelic mutyh mutations and report on novel mutations. Clinical genetics. 2010;78:353–363 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.