Abstract
Excess reactive nitrogen (N) flows from agricultural, suburban, and urban systems to coasts, where it causes eutrophication. Coastal wetlands take up some of this N, thereby ameliorating the impacts on nearshore waters. Although the consequences of N on coastal wetlands have been extensively studied, the effect of the specific form of N is not often considered. Both oxidized N forms (nitrate, NO3−) and reduced forms (ammonium, NH4+) can relieve nutrient limitation and increase primary production. However, unlike NH4+, NO3− can also be used as an electron acceptor for microbial respiration. We present results demonstrating that, in salt marshes, microbes use NO3− to support organic matter decomposition and primary production is less stimulated than when enriched with reduced N. Understanding how different forms of N mediate the balance between primary production and decomposition is essential for managing coastal wetlands as N enrichment and sea level rise continue to assail our coasts.
Keywords: reactive nitrogen, salt marshes, PIE LTER, nitrogen cycling, carbon cycling
The course of human history was dramatically changed when, in 1908, Fritz Haber filed a patent for ammonium (NH4+) production and his contemporary, Carl Bosch, industrialized the process to increase the scale of production. During the tumultuous first half of the twentieth century, this discovery facilitated arms manufacturing, which required extensive supplies of reactive nitrogen (Nr). Even more transformative was its value in fertilizer production, particularly after World War II, which enhanced food availability across the globe. Today, 40%–50% of the human population depends on food grown with fertilizers resulting from the Haber–Bosch process (Smil 2004, Stewart et al. 2005, Erisman et al. 2008), and therefore, increases in human population are tightly coupled with increases in fertilizer use (Erisman et al. 2008). Today, Nr derived via the Haber–Bosch process has more than doubled the annual supply of biologically available nitrogen entering the biosphere (Fowler et al. 2013).
The cascade of Nr from agricultural fields and other sources, through ecosystems, and into coastal waters is well documented (Galloway et al. 2003, Billen et al. 2013). Fertilizer N can be lost from agricultural land through volatilization to the atmosphere that is later deposited as wet or dry deposition (Paerl 1995, Hinga et al. 1991), through leaching into surficial water bodies and into groundwater (Forster et al. 1982), and through harvest and subsequent movement of Nr around the planet in the form of food and feed stocks (Galloway et al. 2008) that ultimately enters wastewater streams. Much of this human-derived Nr eventually finds its way to the N-limited coastal zone, where it increases primary production and, in excess, can lead to eutrophic conditions and anoxia (Nixon 1995, Diaz and Rosenberg 2008). Unfortunately, conversion of Nr back to N2 gas, essentially reversing the Haber–Bosch process, has limited industrial analogs, particular once Nr enters coastal waters. Instead, to prevent eutrophication of the coastal zone, a series of microbial transformations of N must occur for the excess Nr to be returned to inert N2 gas that is then removed from the ecosystem.
Coastal emergent wetlands—principally, salt marshes in the temperate zone—sit between the land and the sea and intercept Nr before it enters open coastal waters (Valiela et al. 2002, Verhoeven et al. 2006, Sousa et al. 2008, Brin et al. 2010, Nelson and Zavaleta 2012). Coastal wetlands can retain or remove some fraction of anthropogenically derived Nr delivered to the coastal zone; however, the amount of Nr retained or removed varies widely, with wetlands in eutrophic systems retaining a smaller fraction of total Nr than wetlands that receive lower Nr inputs (Valiela and Cole 2002). The loss of seagrass beds is considered a sentinel indicator of eutrophic conditions (Orth et al. 2006) and estuaries with large areas of emergent wetlands have greater extents of seagrass beds than similar estuaries with no emergent wetlands because of wetlands’ Nr removal capacity (Valiela and Cole 2002).
The transformations of Nr that are required to ultimately remove N from ecosystems depend on the initial form of the N and on the geochemical conditions present in the environment. There are several mechanisms by which anthropogenic Nr gets delivered to the coastal zone. Nr derived from sewage waste and fertilizers can be transported to estuaries via river (Peierls et al. 1991, Caraco and Cole 1999) and groundwater flows (Valiela et al. 1990, Caraco and Cole 1999, Bowen et al. 2007, Kroeger and Charette 2008), or can be directly discharged from treatment plants into estuarine and coastal waters. This Nr is typically delivered to estuaries as NO3− (Weller and Jordan 2020), with NH4+ often making up less than 10% of Nr as a result of oxygen-dependent nitrification, which converts reduced NH4+ to NO3− through a series of microbially mediated oxidation steps (Ward et al. 2011). Enhanced mobility of NO3− compared with NH4+ and the preferential uptake of NH4+ compared with NO3− by macrophytes, underscores the importance of separately identifying the fates of each form of Nr in coastal systems. Reactive N can also be delivered to coastal systems via atmospheric deposition both directly to the estuarine water body and via deposition on land and subsequent delivery to the estuary via river and groundwater flows. Atmospheric deposition by these mechanisms can account for 10%–40% of total Nr inputs to the coastal zone (Fisher and Oppenheimer 1991, Paerl 1995, Paerl et al. 2002). Historically, oxidized N deposition dominated this flux in eastern North America; however, in recent years, the flux of N oxides decreased as a result of emissions reduction strategies adopted by industrial processes (Lloret and Valiela 2016), whereas the flux of reduced N either increased (largely because of volatilization from animal wastes and fertilizers) or remained constant (Gilliam et al. 2019).
The retention and loss of Nr can occur via multiple pathways (figure 1), including uptake into plant biomass and storage in salt marsh sediments (Valiela and Teal 1979, Drake et al. 2009) and through microbial denitrification, which converts N in its most oxidized form, NO3−, to N2 gas through stepwise reduction (Zumft 1997). This process is largely anaerobic (requiring low oxygen conditions) and heterotrophic (requiring organic carbon as a carbon source; Burgin and Hamilton 2007). Autotrophic (fixing carbon) denitrification also exists, often coupled with reduced iron or sulfur compounds, although its quantitative importance in coastal wetlands is unclear (Rivett et al. 2008). In addition to these denitrification pathways, NO3− can also be converted to NH4+ via microbes that are capable of dissimilatory nitrate reduction to ammonium (DNRA) or anaerobic ammonia oxidation to N2 (annamox). DNRA recycles N within the environment, rather than returning this N to the atmosphere as N2 gas (An and Gardner 2002, Giblin et al. 2013). Organisms capable of DNRA can also be either autotrophic or heterotrophic (Burgin and Hamilton 2007). Annamox also results in loss of Nr (Dalsgaard et al. 2005); however, its importance in coastal wetlands appears limited (Koop-Jakobsen and Giblin 2009). This complex combination of processes, some autotrophic and requiring oxygen and others heterotrophic and requiring anoxic conditions, coupled with different ionic interactions with soil particles (e.g., NH4+ readily binds to clay particles in soils), ultimately dictates the dominant form of Nr in the environment. Understanding the specific pathways involved in the processing of Nr in the coastal zone is therefore essential for predicting the stability of coastal wetlands because shifts in N cycle processes can have simultaneous implications for carbon cycling in these critical carbon-rich habitats (Bulseco et al. 2019).
Figure 1.
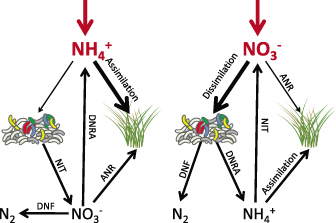
Conceptual diagram illustrating different possible fates of added nitrogen to salt marshes. (a) nitrogen is added in the reduced form of ammonium (NH4+). (b) nitrogen is added in the oxidized form of nitrate (NO3−). We propose that when nitrogen is added as NO3−, it will be predominantly used as an electron acceptor to fuel dissimilatory bacterial respiration rather than to stimulate primary production. ANR = assimilatory nitrate reduction, NIT = nitrification, DNF = denitrification, DNRA = dissimilatory nitrate reduction to ammonium.
When Nr enters coastal salt marshes as NO3−, unlike its reduced forms, it can play two distinct roles (figure 1) with very different consequences at the ecosystem scale. As with NH4+ and organic based fertilizers (figure 1a), NO3− can be used to relieve nutrient limitation and stimulate primary production of vascular plants, benthic algae, and phytoplankton. However, unlike these other forms of N, in the absence of oxygen, it can also be used as an electron acceptor to fuel microbial respiration through denitrification or DNRA (figure 1b). These two opposite outcomes—stimulation of primary production and enhanced decomposition—make it essential that we understand how the forms of Nr entering our coastal waters are being used. If the added NO3− relieves nutrient limitation and supports primary production, it will increase plant biomass, which will facilitate sediment trapping. This increased trapping of sediment, combined with increased peat build up through production of roots and rhizomes, increases the marsh's ability to keep pace with sea level rise (Kirwan et al. 2010, Kirwan and Megonigal 2013, Morris et al. 2013). However, if NO3− is primarily being used as an electron acceptor by the microbial community, this could stimulate microbial respiration and potentially decrease the rate of wetland carbon storage and destabilize marsh creeks (Deegan et al. 2012). By contrast, if the Nr is added in its reduced form, as NH4+, the ecosystem scale outcome will depend on the balance between plant uptake and microbial nitrificiation. If microbes are able to use the NH4+ in nitrification to produce NO3− that is coupled to denitrification, then increased NH4+ should also ultimately stimulate microbial N2 production through coupled nitrification and denitrification; however, if the plants have a higher affinity for available NH4+, then we would expect increased primary production by marsh macrophytes.
Below, we synthesize multiple lines of evidence demonstrating that, when NO3− supply is abundant, primary production rates are lower than are achieved by the addition of comparable supplies of NH4+ and that microbial decomposition is stimulated by NO3− additions. Therefore, it is imperative that we consider not just the quantity but also the form of Nr entering coastal systems to properly manage and mitigate its downstream consequences.
NO3− additions have a smaller effect on primary production of the foundation species Spartina than other forms of Nr
The salt marsh vegetation throughout the East and Gulf Coasts of the United States and in Europe is generally N limited (Valiela and Teal 1979, Kiehl et al. 1997, Tyler et al. 2003) and the addition of exogenous N, particularly when added as NH4+ or organic based fertilizers, typically increases primary production of marsh grasses (figure 2). The foundation species along the East Coast of the United States, Spartina alterniflora and Spartina patens, are both capable of taking up NO3− through their roots (Smith and McLachlan 1979, Mendelssohn 1979b, Cott et al. 2018). S. alterniflora can also take up NO3− through its shoots (Mozdzer et al. 2011), although we could find no studies that examined NO3− uptake via shoots in S. patens. In a field study in North Carolina, nitrate reductase activity was much lower than glutamate dehydrogenase activity (induced by NH4+ availability) across all growth forms of Spartina alterniflora in both the shoots and the roots (Mendelssohn 1979b), suggesting that NH4+ was the preferred nutrient for S. alterniflora growth. A nutrient enrichment experiment indicated that NO3− addition did increase the expression of nitrate reductase in S. alterniflora; however, activity under high ambient NO3− concentrations was still dramatically lower than activity of glutamate dehydrogenase (Mendelssohn 1979b). In addition, foliar uptake of S. alterniflora was around 70% higher for NH4+ and glycine than for NO3− (Mozdzer et al. 2011). S. patens, similarly showed uptake rates of NH4+ that were an order of magnitude higher than NO3− across a range of nutrient concentrations (Cott et al. 2018), further suggesting that, although these Spartina species are capable of NO3− uptake, they prefer N in the form of NH4+.
Figure 2.
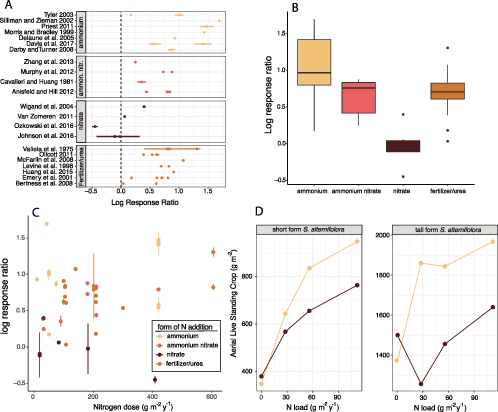
Results of meta-analysis that suggest response to Nr additions in the form of NO3− leads to a smaller positive effect on aboveground biomass of Spartina plants than when other forms of nitrogen enrichment are added. (a) Log response ratios of studies that report response to Nr in enriched compared with reference salt marsh samples separated by the form of N added. (b) Box and whisker plot of the log response ratios reported in panel (a). The center line represents the median response, upper and lower edges of the box represent the upper and lower quartiles, respectively, and the whiskers indicate the highest and lowest values. (c) Log response ratio plotted as a function of the amount of N added. (d) Aboveground biomass by different Spartina ecotypes to the direct addition of NO3− (brown) and NH4+ (yellow) under identical experimental conditions. Source: The data were plotted from table 1 in Mendelssohn (1979a).
We performed a meta-analysis of field studies focusing on Spartina marsh nutrient enrichment to assess whether the form of Nr used in an experiment affected measured outcomes. We included in our study any salt marsh nutrient enrichment experiments where the enriched habitat consisted of predominantly S. alterniflora or S. patens, both the form and quantity of Nr used in the experiment were clearly stated, Nr addition results were presented relative to an unenriched reference, and standard deviation or standard error of the mean and sample size for the response variable were reported. There were too few studies documenting the response of belowground vegetation so we focused on the response of aboveground Spartina biomass to Nr enrichment (figure 2).
Twenty-two studies from the Western Atlantic (n = 17), the Gulf of Mexico (n = 3) in the United States, and China (n = 2) clustered into four different types of added Nr: NH4+ (n = 7), NH4NO3 (n = 4), other forms of NO3− (n = 4), and organic N, either in the form of pelletized sewage sludge or organic fertilizer or as urea (n = 7). We used the “escalc” function in the metafor v.2.1–0 R package (Viechtbauer 2010) to calculate the mean and variance in effect size using ROM, the log transformed ratio of the means (Hedges et al. 1999, Lajeunesse 2011), as the effect size measure while specifying the means, standard deviations, and sample sizes from each study.
We found that added Nr in the form of NO3− (excluding Nr that was added as NH4NO3) had a smaller effect size on plant biomass than other forms of Nr (figure 2a). When studies were aggregated together by the form of Nr added (figure 2b) the mean effect size was significantly less (ANOVA, F(3,40) = 9.89, p < .01) in the NO3− only additions (Tukey's posthoc test: NO3− versus NH4+, p ≤ .01; NO3− versus NH4NO3, p = .044; NO3− versus organic fertilizer N or urea, p ≤ .01). There was no relationship between the quantity of N added and the effect size across all treatments (figure 2c), suggesting that the muted responses observed in NO3− addition experiments were not the result of a lower overall amount of Nr added in the enrichment experiments, but rather, it was the form of N that played a consequential role.
Mendelssohn (1979a) performed the only study that directly compared the change in Spartina alterniflora aboveground biomass when plants were grown separately with NO3− or NH4+ as the added Nr source. This study was not included in our meta-analysis because no error was reported; however, the results are consistent with our hypothesis of a muted response by S. alterniflora to Nr enrichment when it is added as NO3− (figure 2d). Mendelssohn measured live standing crop of both the tall ecotype and the short ecotype of S. alterniflora from the East Coast (Walden Creek Marsh, North Carolina) under different nutrient regimes. The experiment used a 4 × 2 × 2 factorial design with four N application rates ranging from 0 to 112 grams per square meter per year of added Nr, two different application methods (band, where Nr was injected into the sediment, and broadcast, where Nr was placed on the surface sediments), and two different N forms, (NH4)2SO4 and NaNO3. For both the short and tall ecotypes of Spartina alterniflora when Nr was added as (NH4)2SO4 there was a greater increase in growth than when it was added as NaNO3 (figure 2d) We show only the results of band delivery in the present article but the response to the broadcast delivery method were comparable (Mendelssohn 1979a). Our meta-analysis and Mendelssohn's direct test indicate that Spartina biomass accumulation is lower with NO3− enrichment, which could have consequences for the long-term sustainability of marshes in the face of rising sea levels.
Whole creek 15N-NO3− enrichment experiments support rapid uptake by microalgae and little NO3− uptake by Spartina
In unvegetated areas of salt marshes and even in understory areas of the marsh where there is sufficient light penetration, benthic microalgae, along with macrophytes and denitrifying bacteria (Sundback and Miles 2000), use Nr. Benthic diatoms are capable of NO3− uptake and storage (Lomas and Glibert 2000); however, responses to the addition of Nr by benthic microalgae vary widely, depending on the study (Sullivan and Currin 2002). Whole ecosystem additions of enriched 15N-NO3− in saltmarshes quickly and consistently result in highly labeled benthic algae (Hughes et al. 2000, Tobias et al. 2003a, Tobias et al. 2003b, Drake et al. 2009, Galván et al. 2011), suggesting uptake of NO3−. However, the measured uptake kinetics of benthic microalgae were consistently higher when provided ammonium, compared with NO3− in estuarine sediments of the Nakdong Estuary on the Korean Peninsula (Longphuirt et al. 2009). Our 13-year salt marsh NO3− enrichment experiment, referred to as the TIDE project, showed that the addition of NO3− did not appreciably increase standing stock biomass of benthic microalgae (Deegan et al. 2007), although high grazing could have masked an increase in algal productivity (Galván et al. 2011, Pascal and Fleeger 2013).
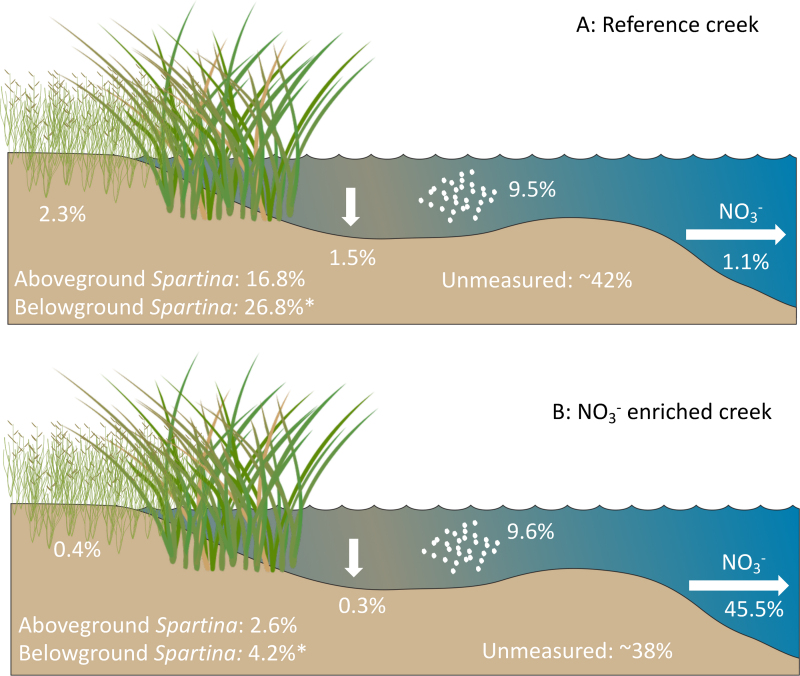
The partitioning of NO3− between salt marsh macrophytes, benthic microalgae, and microbial dissimilatory nitrate respiration pathways can partly be disentangled using whole marsh 15N-NO3− addition experiments (Drake et al. 2009). As a part of the TIDE project, we performed a whole-creek marsh 15N-NO3− addition experiment in a creek that was amended to 70 micromolar (μM) NO3− dissolved into flooding tidal waters on every tide for 2 years and in reference creeks that had ambient (less than 5 μM) NO3− concentrations. 15N-NO3− was added to both creeks for 5 days in midsummer (23–28 July 2005). In the NO3− enriched creek, enough 15N-NO3− was added to maintain creek water at 650‰ enrichment while keeping the creek NO3− concentration at approximately 70 μM. In the reference creek we maintained a target enrichment of 1000‰, which resulted in a 3%–11% increase in the creek NO3− concentration (Drake et al. 2009). The reference creek was able to retain 98.8% of the added 15N-NO3− (figure 3a). Aboveground plant biomass accumulated approximately 17% of the isotope label and belowground biomass was estimated to take up an additional approximately 25%, although belowground accumulation was not directly measured. The sediment pool, including benthic microalgae, took up less than 2% of the added NO3−. After all the measured pools were accounted for, 42% of the added NO3− in the reference marsh remained unaccounted for and was assumed to be lost via microbial denitrification. In the NO3− enriched marsh, 54.5% of added NO3− was retained in the plant and sediment pools. Although the percentage retention in the enriched marsh was lower than in the reference marsh, the total mass of NO3− retained in the system was higher, because of an overall larger mass of NO3− added during the experiment. In the enriched creek, the plants and benthic microalgae retained less than 10% of the added NO3− and approximately 38% was unaccounted for and assumed to be lost via microbial denitrification. The remainder was exported from the creek in the tidal waters. These enrichment experiments occurred toward the latter part of the growing season of Spartina, when biomass accumulation had slowed. It remains to be seen whether similar partitioning of added NO3− would be observed during times of peak Spartina growth. These results further support our hypothesis that salt marsh vegetation and the benthic microalgal communities use a small amount of added NO3− compared with presumed dissimilatory NO3− respiration performed by the microbial community.
Figure 3.
Mass balance of 15NO3− added in low dose to an unenriched reference salt marsh creek (a) and in high dose to a NO3−enriched salt marsh creek (b) during a paired 15N isotope enrichment experiment (Drake et al. 2009). The reference marsh retained 98.8% of added NO3−, compared with 54.5% in the enriched marsh. The percentages are the percentage of added NO3− found in each marsh pool after 5 days of dosing. NO3− that was unaccounted for was assumed to be lost via dissimilatory microbial processes such as denitrification. Asterisk indicates that the value was estimated and not directly measured. More details on the experimental design and measurements can be found in Drake and colleagues (2009). Source: The images of marsh vegetation were compiled from the IAN symbol repository.
Both field and controlled laboratory experiments support microbial use of added NO3−
The whole creek 15N- NO3− isotope enrichment experiment, both in enriched and reference creeks, had high proportions of 15N-NO3− that were unaccounted for and that were assumed to be lost via microbial denitrification (Drake et al. 2009); however, denitrification was not directly measured. Additional work by Koop-Jakobsen and Giblin (2010) measured the rates of DNRA, denitrification that is coupled with nitrification, direct denitrification, and rhizosphere associated denitrification in specific subhabitats (creek sediments and high marsh platform) within nutrient enriched and unamended creeks. During flooding tides, the addition of NO3− stimulated direct denitrification, which was nearly twentyfold higher on the fertilized marsh platform than at the reference site (figure 4a). DNRA on the fertilized platform was also stimulated although it was only a fourfold stimulation. In contrast, there was no stimulation of coupled denitrification in either the surface sediments or the rhizosphere (figure 4a).
Figure 4.
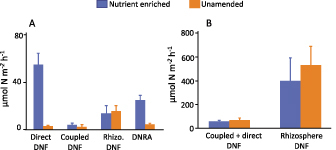
Field- and lab-based assessments of the fate of added NO3− in salt marsh sediments. (a) Direct denitrification, coupled denitrification, rhizosphere (rhizo.) associated denitrification and dissimilatory reduction of nitrate to ammonium (DNRA) rates measured on the marsh platform in NO3− enriched and unamended marshes (adapted from Koop-Jakobsen and Giblin 2010). (b) rates of coupled plus direct denitrification and rhizosphere denitrification in cores collected from NO3− and unamended marshes when exposed to comparable supplies of exogenous NO3−.
The major reason for this increase in direct denitrification appears to be a direct and rapid response to added NO3−. Pore water measurements showed that the flooding NO3− did not reach the rhizosphere in the high marsh and therefore rates were similar between reference and enriched sites. When sediments collected from reference sites were amended with NO3− in whole core incubations, denitrification rates increased and did not differ from sediments in enriched sites when both treatments received comparable amounts of NO3− (figure 4b). Similarly, when NO3− was added to the rhizosphere of sediments from the reference and the enriched sites, there was a large stimulation in denitrification rates in sediments from both sites. Therefore, in all cases when NO3− was directly added to marsh sediments, both to the rhizosphere and to the overlying water in intact cores, there was a rapid increase in microbial respiration of NO3− through denitrification. In situ measurements of ecosystem respiration (Geoghegan et al. 2018) and soil respiration (Wigand et al. 2018) were also significantly higher in the nutrient enriched creeks, providing further evidence of NO3− stimulated microbial processes. This suggests that salt marsh sediments have populations of microbes capable of using NO3− within hours of it being added and implies that there is a sufficient supply of biologically available salt marsh organic matter to support this NO3− respiration.
To explore the capacity of salt marsh microbes to use NO3− to decompose organic matter over longer time periods, we used a flow through reactor (FTR) approach (Bulseco et al. 2019). In an FTR, seawater flows through reactors filled with homogenized marsh sediment and peat, half of which were enriched for approximately 90 days with 15N-NO3−, and we measured total NO3− reduction on the basis of the difference between the NO3− concentration entering the reactor and the concentration leaving the reactor. Simultaneously, we measured production of 30N2 (denitrification) and production of 15NH4 (DNRA) in sediments collected from the top 5 centimeters (cm), 10–15 cm, and 20–25 cm depth from a Spartina marsh (table 1). Regardless of the depth from which the sediments were collected, NO3− addition resulted in a stimulation of decomposition (Bulseco et al. 2019). When we added enough NO3− that it was never limiting, denitrification was the dominant NO3− loss process (table 1). The combined rates of denitrification and DNRA accounted for the total amount of NO3− that was consumed by the reactors (table 1), further supporting our supposition that seemingly biologically unavailable salt marsh peat can support substantial rates of denitrification.
Table 1.
Total nitrate reduction, denitrification (DNF) and dissimilatory nitrate reduction to ammonium (DNRA) integrated across the duration of the flow through reactor experiment.
| Nitrate reduction (mmol per cm3) | DNF (mmol per cm3) | DNRA (mmol per cm3) | DNRA + DNF (mmol per cm3) | ||||||
| Depth | Mean (M) | Standard deviation (SD) | M | SD | M | SD | M | SD | Percentage of nitrate reduction |
| Shallow (0–5 cm) | 87.5 | 7.4 | 70.6 | 1.7 | 10.2 | 0.6 | 80.9 | 1.8 | 92 |
| Mid (10–15 cm) | 61.3 | 4.1 | 58.1 | 3.7 | 4.8 | 1.4 | 63 | 4.0 | 102 |
| Deep (20–25 cm) | 70.9 | 20.4 | 55.2 | 10.3 | 3.6 | 0.9 | 58.8 | 10.3 | 82 |
Note: The sum of DNF and DNRA accounted for between 82%–102% of the total amount of nitrate reduced during the experiment. The data were derived from Bulseco and colleagues (2019). Abbreviations: cm3, cubic centimeters; mmol, millimoles.
NO3− respiration liberates more free energy for microbes than respiration via sulfate reduction or fermentation, two of the most important organic matter decomposition processes in salt marsh sediments (Howarth 1984). Therefore, we hypothesized that the addition of NO3− would not only stimulate additional decomposition, but also that there would be a pool of complex organic matter that could be decomposed in the presence of NO3− that would not be decomposed by microbes using less energetically favorable electron acceptors, such as sulfate (Bulseco et al. 2019). Indeed, deeper sediments showed less stimulation of dissolved inorganic carbon (DIC) production than surface sediments, indicating that the quality of the carbon in salt marsh sediments does differ; however, at all depths, respiration was stimulated by NO3− addition (Bulseco et al. 2019).
We also measured the byproducts of respiration in the flow through reactor experiment and calculated the ratio of DIC:NH4+ produced over time (figure 5). We found that in surface sediments, where newly deposited biologically available carbon is abundant, there was little difference in the DIC:NH4+ ratio between reactors that received NO3− and those that did not. However, in the mid-depth and deep sediments, where the organic matter is likely less biologically available and more complex, there was an increase in the DIC:NH4+ ratio when NO3− was added compared with the unamended reactors. This suggests that the addition of NO3− allowed the microbes to access a pool of organic matter that was not accessed in the unamended reactors. In the absence of added NO3−, this carbon would remain stable, which has implications for our understanding of carbon storage in eutrophic systems. If excess NO3− in coastal systems allows for enhanced decomposition of more complex carbon it could slow the rate of carbon storage in these systems, relative to systems that do not receive excess NO3− supply.
Figure 5.
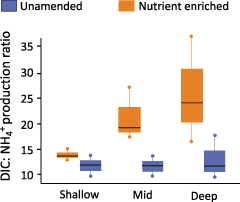
DIC:NH4+ production ratio, as a proxy for the complexity of the organic matter being decomposed. A high ratio indicates a more complex carbon source. Horizontal black bars indicate the median values and whiskers represent the upper and lower quartiles. Source: Adapted from Bulseco and colleagues (2019).
To confirm the microbial role in NO3− use via enhanced denitrification or DNRA and to test our supposition that microbes exposed to high concentrations of NO3− are able to decompose more complex organic matter, we used metagenomics to link our rate measurements to their underlying microbial mechanisms. Analysis of sediment metagenomics is complex because the tremendous diversity of sediment microbes makes deciphering patterns in genetic changes challenging. Previous research on the microbial community structure in the TIDE project indicated that although NO3− increased microbial leucine uptake (a proxy for microbial production) in bare sediment that receives sufficient light to promote increased microalgal production, there did not appear to be a stimulation of microbial productivity in more heavily vegetated regions of the marsh (Bowen et al. 2009a). Even in regions where leucine uptake was enhanced, the increase in leucine uptake did not translate into shifts in the overall microbial community structure, which was remarkably consistent over a decade of nutrient enrichment (Bowen et al. 2009b, 2011). We did observe large shifts in the active bacterial community, including shifts in bacterial taxa known to denitrify (Kearns et al. 2016), and shifts in putative fungal denitrifiers (Kearns et al. 2019), which suggests that microbial communities using NO3− as an electron acceptor may be able to increase respiration using NO3−, without leading to a whole scale shift in the community structure.
In light of these results, it is not surprising that we were unable to detect a strong signal of nutrient enrichment in the microbial metagenomes from our field experiment (figure 6a). In the FTR experiments, however, where NO3− was added at higher concentrations and in a continuous manner, we saw broad shifts in the metagenomes (figure 6b) that are consistent with the biogeochemical rate measurements (Bulseco et al. 2020). Many genes involved in central carbon metabolism were enhanced in the NO3− addition (figure 6c, supplemental table S1), including increases in the Entner–Doudoroff pathway for the generation of pyruvate from glucose, and decarboxylates that harness that pyruvate for cellular respiration, supporting the hypothesis that the addition of NO3− allows the microbial community to access otherwise inaccessible organic matter.
Figure 6.
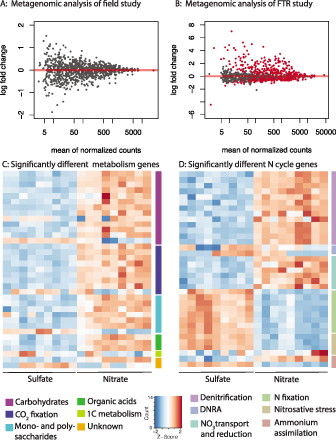
Metagenomic evidence for increased microbial respiration during NO3− addition in field and laboratory experiments. (a) Log-fold differences in gene abundance between NO3− enriched and unenriched salt marsh surface sediments collected from the TIDE nutrient enrichment experiment. (b) Log-fold differences in gene abundance in FTRs between NO3− amended reactors and reactors receiving only seawater. Red points indicate genes that are significantly different between the two treatments. (c) Heat map indicating the 35 biggest differences between amended and unamended FTRs for genes involved in carbon metabolism. (d) Heat map indicating the 35 biggest differences between amended and unamended FTRs for genes involved in the nitrogen cycle. Source: The data used in panels (c) and (d) were adapted from Bulseco and colleagues (2020).
Surprisingly, we also observed an increase in genes associated with CO2 fixation as a result of nutrient enrichment, suggesting that added NO3− could also promote autotrophic denitrification pathways (Bulseco et al. 2020). Many of the genes responsible for the cycling of nitrogen, including all the genes found in the denitrification pathway, were also significantly higher in the NO3− amended FTRs, compared with the unamended reactors (figure 6d, table S1). By contrast, subsystems involved in fermentation and other low energy producing metabolisms were more abundant in the unamended treatment (figure 6c) as were genes responsible for N fixation (figure 6d). The metagenomics findings from our FTR experiment are consistent with the biogeochemical rates we measured (Bulseco et al. 2020) and provide a mechanistic link that connects microbial genetics to the NO3− loss and DIC production we observed under NO3− addition.
Conclusions
Reactive nitrogen enrichment of the coastal zone is well documented, but frequently, the form of N is overlooked. Our results suggest that understanding the form of N that is biologically available in coastal systems is critically important for managing coastal resources. Most anthropogenically derived N delivered to the coastal zone is in its oxidized form, as NO3−. Our rate measurements, both in the field and in the laboratory, show that NO3− is being used as an electron acceptor to fuel decomposition, and that this overshadows its role as a nutrient to support growth of plant biomass. Our results suggest that microbes can access available NO3− and use it as an electron acceptor to respire organic matter that might have otherwise been stored. This process could potentially decrease the carbon sink capacity of marshes because of enhanced decomposition and limit effects on primary production needed to facilitate sediment trapping and offset increased decomposition.
In all likelihood, the use of NO3− in coastal marsh sediments is not a winner take all scenario, where 100% of the resource goes either to the microbes or to the plants, but rather, the ecosystem outcome is a function of the relative responses of the two groups of organisms. Several unknowns need resolution before predictive models (e.g., the Marsh Equilibrium Model, Morris and Bowden 1986, Morris et al. 2002) can accurately incorporate nutrient supply into their algorithms to predict marsh persistence relative to sea level rise. First, with a carbon to N ratio of around 30, marsh vegetation requires a fairly small amount of N to fix a fairly large amount of carbon and it remains to be seen whether losing a portion of that NO3− to the microbial community materially alters the productivity or annual storage by vascular plants. Second, we need to better parameterize whether variations in the timing, supply, and duration of use of NO3− or NH4+ by plants, microalgae, and microbes has long-term consequences for marsh carbon storage. In particular, the variation in growing season length and phenology, which may drive plant nutrient uptake, varies with latitude and could affect response to increasing supply of NO3− and NH4+. Third, we need a better understanding of how aboveground and belowground biomass is partitioned and how these factors feedback on sediment trapping and storage when N is supplied as NO3− rather than as NH4+. Finally, our genomic data suggest that chemoautotrophic metabolisms could play a larger than expected role in carbon fixation in nutrient enriched marshes; however, the extent of this needs to be better parameterized. Understanding these critical unknowns will be essential for predicting marsh carbon storage in a high nutrient world.
Supplementary Material
Acknowledgments
This work was supported by the following funding sources: National Science Foundation (NSF) grant no. DEB 1902712 to LAD, JLB, DSJ, and TJM; NSF grant no. DEB 1902695 to AEG; NSF grant no. DEB 1902704 to JAN; NSF grant no. DEB 1354214 to TJM; NSF grant no. DEB 1350491 to JLB; NSF grant no. OCE 1637630 to AEG and LAD; and additional funding from the Dorr Foundation, the Department of the Interior Northeast Climate Science Center (grant no. DOI G12AC00001), and a Bullard Fellowship (Harvard University) to LAD and from the National Academies of Science, Medicine, and Engineering Gulf Research Program to JAN. Resources purchased with funds from the NSF Biological Field Stations and Marine Laboratories program (grant no. DBI 1722553, to Northeastern University) were used to generate the data for the manuscript. Initial conversations on the effects of nutrient enrichment in marshes with Scott Warren and Bruce Peterson were critical in informing the work described in the manuscript. Sam Kelsey and Jane Tucker contributed to much of the N cycling biogeochemistry; Caitlin Bauer, Frankie Leach, Paige Weber, Emily Geoghegan and Sophie Drew assisted with field work; and Joe Vineis assisted with metagenomic analysis. This is contribution 3941 from the Virginia Institute of Marine Science. The data were compiled from multiple published sources. Links to published data can be found here: https://pie-lter.ecosystems.mbl.edu/data. The sequence data used to derive figure 6 are publicly available on the MG-RAST website under project number mgp84173.
Author Biographical
Jennifer Bowen (je.bowen@northeastern.edu) is an associate professor and Anna Murphy is a postdoctoral scholar at Northeastern University's Marine Science Center, in Nahant, Massachusetts, and a senior scientist at INSPIRE Environmental, in Newport, Rhode Island. Linda Deegan is a senior scientist and Hillary Sullivan is a research assistant at the Woodwell Climate Research Center (formerly, the Woods Hole Research Center), in Falmouth, Massachusetts. Deegan leads the TIDE project, the long-term nutrient enrichment experiment from which much of these results derive. Anne Giblin is the director of the Plum Island Ecosystems LTER, on Plum Island, Massachusetts, and Ashley Bulseco was a postdoctoral scholar at the Marine Biological Laboratory, in Woods Hole, Massachusetts, and is now an assistant professor of Marine Science at Eckerd College, in St. Petersburg, Florida. David Samuel Johnson is an assistant professor at the Virginia Institute of Marine Science, at William and Mary, in Gloucester Point, Virginia. Thomas Mozdzer is an associate professor at Bryn Mayr College, in Bryn Mayr, Pennsylvania. James Nelson is an assistant professor at the University of Louisiana at Lafayette.
Contributor Information
Jennifer L Bowen, Northeastern University's Marine Science Center, Nahant, Massachusetts, and a senior scientist at INSPIRE Environmental, Newport, Rhode Island.
Anne E Giblin, Marine Biological Laboratory, Woods Hole, Massachusetts, and is now an assistant professor of Marine Science at Eckerd College, St. Petersburg, Florida.
Anna E Murphy, Northeastern University's Marine Science Center, Nahant, Massachusetts, and a senior scientist at INSPIRE Environmental, Newport, Rhode Island.
Ashley N Bulseco, Marine Biological Laboratory, Woods Hole, Massachusetts, and is now an assistant professor of Marine Science at Eckerd College, St. Petersburg, Florida.
Linda A Deegan, Woodwell Climate Research Center (formerly, the Woods Hole Research Center), in Falmouth, Massachusetts. Deegan leads the TIDE project, the long-term nutrient enrichment experiment from which much of these results derive.
David S Johnson, Virginia Institute of Marine Science, William and Mary, Gloucester Point, Virginia.
James A Nelson, University of Louisiana, Lafayette.
Thomas J Mozdzer, Bryn Mayr College, Bryn Mayr, Pennsylvania.
Hillary L Sullivan, Woodwell Climate Research Center (formerly, the Woods Hole Research Center), in Falmouth, Massachusetts. Deegan leads the TIDE project, the long-term nutrient enrichment experiment from which much of these results derive.
References cited
- An S, Gardner WS.. 2002. Dissimilatory nitrate reduction to ammonium (DNRA) as a nitrogen link, versus denitrification as a sink in a shallow estuary (Laguna/Madre/Baffin Bay, Texas). Marine Ecology Progress Series 237: 41–50. [Google Scholar]
- Anisfeld SC, Hill TD.. 2012. Fertilization effects on elevation change and belowground carbon balance in a Long Island Sound tidal marsh. Estuaries and Coasts 35: 201–211. [Google Scholar]
- Bertness MD, Crain C, Holdredge C, Sala N. 2008. Eutrophication and consumer control of New England salt marsh primary productivity. Conservation Biology 22: 131–139. [DOI] [PubMed] [Google Scholar]
- Billen G, Garnier J, Lassaletta L. 2013. The nitrogen cascade from agricultural soils to the sea: Modelling nitrogen transfers at regional watershed and global scales. Philosophical Transactions of the Royal Society B 368: 20130123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen JL, Kroeger KD, Tomasky G, Pabich WJ, Cole ML, Carmichael RH, Valiela I. 2007. A review of land-sea coupling by groundwater discharge of nitrogen to New England estuaries: Mechanisms and effects. Applied Geochemistry 22: 175–191. [Google Scholar]
- Bowen JL, Crump BC, Deegan LA, Hobbie JE. 2009a. Increased supply of ambient nitrogen has minimal effect on salt marsh bacterial production. Limnology and Oceanography 54: 713–722. [Google Scholar]
- Bowen JL, Crump BC, Deegan LA, Hobbie JE. 2009b. Salt marsh sediment bacteria: Their distribution and response to external nutrient inputs. ISME Journal 3: 924–934. [DOI] [PubMed] [Google Scholar]
- Bowen JL, Ward BB, Morrison HG, Hobbie JE, Valiela I, Deegan LA, Sogin ML. 2011. Microbial community composition in sediments resists perturbation by nutrient enrichment. ISME Journal 5: 1540–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brin LD, Valiela I, Goehringer D, Howes B. 2010. Nitrogen interception and export by experimental salt marsh plots exposed to chronic nutrient addition. Marine Ecology Progress Series 400: 3–17. [Google Scholar]
- Bulseco AN, Giblin AE, Tucker J, Murphy AE, Sanderman J, Hiller‐Bittrolff K, Bowen JL. 2019. Nitrate addition stimulates microbial decomposition of organic matter in salt marsh sediments. Global Change Biology 25: 3224–3241. [DOI] [PubMed] [Google Scholar]
- Bulseco AN, Vineis JH, Murphy AE, Spivak AC, Giblin AE, Tucker J, Bowen JL. 2020. Metagenomics coupled with biogeochemical rates measurements provide evidence that nitrate addition stimulates respiration in salt marsh sediments. Limnology and Oceanography 65: S321–S339. [Google Scholar]
- Burgin AJ, Hamilton SK.. 2007. Have we overemphasized the role of denitrification in aquatic ecosystems? Frontiers in Ecology and the Environment 5: 89–96. [Google Scholar]
- Caraco N, Cole JJ.. 1999. Human impact on nitrate export: An analysis using major world rivers. Ambio 28: 167–170. [Google Scholar]
- Cavalieri AJ, Huang AHC.. 1981. Accumulation of proline and glycine betaine in Spartina alterniflora Loisel. in response to NaCl and nitrogen in the marsh. Oecologia 49: 224–228. [DOI] [PubMed] [Google Scholar]
- Cott GM, Caplan JS, Mozdzer TJ. 2018. Nitrogen uptake kinetics and saltmarsh plant responses to global change. Scientific Reports 8: 5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard T, Thamdrup B, Canfield DE. 2005. Anaerobic ammonium oxidation (anammox) in the marine environment. Research in Microbiology 156: 457–464. [DOI] [PubMed] [Google Scholar]
- Darby FA, Turner RE.. 2008. Below- and aboveground biomass of Spartina alterniflora: Response to nutrient addition in a Louisiana salt marsh. Estuaries and Coasts 31: 326–334. [Google Scholar]
- Davis J, Currin C, Morris JT. 2017. Impacts of fertilization and tidal inundation on elevation change in microtidal, low relief salt marshes. Estuaries and Coasts 40: 1677–1687. [Google Scholar]
- Deegan LA, et al. 2007. Susceptibility of salt marshes to nutrient enrichment and predator removal. Ecological Applications 17: S42–S63. [Google Scholar]
- Deegan LA, Johnson DS, Warren RS, Peterson BJ, Fleeger JW, Fagherazzi S, Wollheim WM. 2012. Coastal eutrophication as a driver of salt marsh loss. Nature 490: 388–392. [DOI] [PubMed] [Google Scholar]
- DeLaune RD, Pezeshki RD, Jugsujinda A. 2005. Impact of Mississippi River freshwater reintroduction on Spartina patens marshes: Response to nutrient input and lowering of salinity. Wetlands 25: 155–161. [Google Scholar]
- Diaz RJ, Rosenberg R.. 2008. Spreading dead zones and consequences for marine ecosystems. 2008. Science 321: 926–929. [DOI] [PubMed] [Google Scholar]
- Drake DC, Peterson BJ, Galván KA, Deegan LA, Hopkinson C, Johnson JM, Koop-Jakobsen K, Lemay LE, Picard C. 2009. Salt marsh ecosystem biogeochemical responses to nutrient enrichment: A paired 15N tracer study. Ecology 90: 2535–2546. [DOI] [PubMed] [Google Scholar]
- Emery NC, Ewanchuk PJ, Bertness MD. 2001. Competition and salt marsh plant zonation: Stress tolerators may be dominant competitors. Ecology 82: 2471–2485. [Google Scholar]
- Erisman JW, Sutton MA, Galloway J, Klimont Z, Winiwarter W. 2008. How a century of ammonia synthesis changed the world. Nature Geoscience 1: 636–639. [Google Scholar]
- Fisher DC, Oppenheimer M.. 1991. Atmospheric nitrogen deposition and the Chesapeake Bay estuary. Ambio 20: 102–108. [Google Scholar]
- Forster SSD, Cripps AC, Smith-Carington A. 1982. Nitrate leaching to groundwater. Philosophical Transactions of the Royal Society B 296: 21 10.1098/rstb.1982.0021. [DOI] [Google Scholar]
- Fowler D, et al. 2013. . The global nitrogen cycle in the twenty-first century. Philosophical Transactions of the Royal Society B 368: 20130164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway JN, Aber JD, Erisman JW, Seitzinger SP, Howarth RW, Cowling EB, Cosby BJ. 2003. The Nitrogen Cascade. BioScience 53: 341–356. [Google Scholar]
- Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA. 2008. Transformations of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 320: 889–892. [DOI] [PubMed] [Google Scholar]
- Galván K, Fleeger JW, Peterson B, Drake D, Deegan LA, Johnson DS. 2011. Natural stable isotopes and dual isotope tracer additions help to resolve resources supporting a saltmarsh food web. Journal of Experimental Marine Biology and Ecology 410: 1–11. [Google Scholar]
- Geoghegan EK, Caplan JS, Leech FN, Weber PE, Bauer CE, Mozdzer TJ. 2018. Nitrogen enrichment alters carbon fluxes in a New England salt marsh. Ecosystem Health and Sustainability 4: 277–287. [Google Scholar]
- Giblin AE, Tobias CR, Song B, Weston N, Banta GT. 2013. The importance of dissimilatory nitrate reduction to ammonium (DNRA) in the nitrogen cycle of coastal ecosystems. Oceanography 26: 124–131. [Google Scholar]
- Gilliam FS, Burns DA, Driscoll CT, Frey SD, Lovett GM Watmough SA. 2019. Decreased atmospheric deposition in eastern North America: Predicted responses of forest ecosystems. Environmental Pollution 244: 560–574. [DOI] [PubMed] [Google Scholar]
- Hedges LV, Gurevitch J, Curtis PS. 1999. The meta-analysis of response rations in experimental ecology. Ecology 80: 1150–1156. [Google Scholar]
- Hinga KE, Keller AA, Oviatt CA. 1991. Atmospheric deposition and nitrogen input to coastal waters. Ambio 20: 256–260. [Google Scholar]
- Howarth RW. 1984. The ecological significance of sulfur in the energy dynamics of salt marsh and coastal marine sediments. Biogeochemistry 1: 5–27. [Google Scholar]
- Huang J, Xu X, Wang M, Nie M, Qiu S, Wang Q, Quan Z, Xiao M, Li B. 2015. Responses of soil nitrogen fixation to Spartina alterniflora invasion and nitrogen addition in a Chinese salt marsh. Scientific Reports 6: 20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JE, Deegan LA, Peterson BJ, Holmes RM, Fry B. 2000. Nitrogen flow through the food web in the oligohaline zone of a New England estuary. Ecology 81: 433–452. [Google Scholar]
- Johnson DJ, Warren RS, Deegan LA, Mozdzer TJ. 2016. Saltmarsh plant responses to eutrophication. Ecological Applications 26: 2649–2661. [DOI] [PubMed] [Google Scholar]
- Kearns PJ, Angell JH, Howard EM, Deegan LA, Stanley RHR, Bowen JL. 2016. Nutrient enrichment induces dormancy and decreases diversity of active bacteria in salt marsh sediments. Nature Communications 7: 12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns PJ, Bulseco AN, Hoyt H, Angell JH, Bowen JL. 2019. Nutrient enrichment alters salt marsh fungal communities and promotes putative fungal denitrifiers. Microbial Ecology 77: 358–369. [DOI] [PubMed] [Google Scholar]
- Kiehl K, Esselink P, Bakker JP. 1997. Nutrient limitation and plant species composition in temperate salt marshes. Oecologia 111: 325–330. [DOI] [PubMed] [Google Scholar]
- Kirwan ML, Megonigal JP.. 2013. Tidal wetland stability in the face of human impacts and sea-level rise. Nature 504: 53–60. [DOI] [PubMed] [Google Scholar]
- Kirwan ML, Guntenspergen GR, D'Alpaos A, Morris JT, Mudd SM, Temmerman S. 2010. Limits on the adaptability of coastal marshes to rising sea level. Geophysical Research Letters 37: L23401. [Google Scholar]
- Koop-Jakobsen K, Giblin AE.. 2009. Annamox in tidal marsh sediments: The role of vegetation, salinity, nitrogen loading and marsh vegetation. Estuaries and Coasts 32: 238–245. [Google Scholar]
- Koop-Jakobsen K, Giblin AE.. 2010. The effect of increased nitrate loading on nitrate reduction via denitrification and DNRA in salt marsh sediments Limnology and Oceanography 55: 789–802. [Google Scholar]
- Kroeger KD, Charette MA.. 2008. Nitrogen biogeochemistry of submarine groundwater discharge. Limnology and Oceanography 53: 1025–1039. [Google Scholar]
- Lajeunesse MJ. 2011. On the meta-analysis of response ratios for studies with correlated and multi-group designs. Ecology 92: 2049–2055. [DOI] [PubMed] [Google Scholar]
- Levine JM, Brewer JS, Bertness MD. 1998. Nutrients, competition and plant zonation in a New England salt marsh. Journal of Ecology 86: 285–292. [Google Scholar]
- Lomas MW, Glibert PM.. 2000. Comparisons of nitrate uptake, storage, and reduction in marine diatoms and flagellates. Journal of Phycology 36: 903–913. [Google Scholar]
- Longphuirt SN, Lim J-H, Leynaert A, Claquin P, Choy E-J, Kang C-K, An S. 2009. Dissolved inorganic nitrogen uptake by intertidal microphytobenthos: Nutrient concentrations, light availability and migration. Marine Ecology Progress Series 379: 33–44. [Google Scholar]
- Lloret J, Valiela I.. 2016. Unprecedented decrease in deposition of nitrogen oxides over North America: The relative effects of emission controls and prevailing air-mass trajectories. Biogeochemistry 129: 165–180. [Google Scholar]
- McFarlin CR, Brewer JS, Buck TL, Pennings S. 2008. Impact of fertilization on a salt marsh food web in Georgia. Estuaries and Coasts 31: 313–325. [Google Scholar]
- Mendelssohn IA. 1979a. The influence of nitrogen level, form, and application method on the growth response of Spartina alterniflora in North Carolina. Estuaries 2: 106–112. [Google Scholar]
- Mendelssohn IA. 1979b. Nitrogen metabolism in the height forms of Spartina alterniflora in North Carolina. Ecology 60: 574–584. [Google Scholar]
- Morris JT, Bowden WB.. 1986. A mechanistic numerical model of sedimentation, mineralization, and decomposition for marsh sediments. Soil Science Society of America Journal 50: 96–105. [Google Scholar]
- Morris JT, Sundareshwar PV, Nietch CT, Kjerfve B, Cahoon DR. 2002. Response of coastal wetlands to rising sea level. Ecology 83: 2869–2877. [Google Scholar]
- Morris JT, Sundberg K, Hopkinson CS. 2013. Salt marsh primary production and its responses to relative sea level and nutrients in estuaries at Plum Island, Massachusetts, and North Inlet, South Carolina USA. Oceanography 26: 78–84. [Google Scholar]
- Mozdzer TJ, Kirwan M, McGlathery KJ, Zieman JC. 2011. Nitrogen uptake by the shoots of smooth cordgrass Spartina alterniflora. Marine Ecology Progress Series 433: 43–52. [Google Scholar]
- Murphy SM, Wimp GM, Lewis D, Denno RF. 2012. Nutrient presses and pulses differentially impact plants, herbivores, detritivores and their natural enemies. PLOS ONE 7: e43929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JL, Zavaleta ES.. 2012. Salt marshes as a coastal filter for the oceans: Changes I function with experimental increases in nitrogen loading and sea-level rise. PLOS ONE 7: e38558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon SW. 1995. Coastal marine eutrophication: A definition, social causes and future concerns Ophelia 41: 199–219. [Google Scholar]
- Olcott CA. 2011. Impacts of Nitrogen Addition on the Monthly Above- and Belowground Production of Spartina alterniflora in a Virginia Marsh. Master's thesis University of Virginia, Charlottesville, Virginia. [Google Scholar]
- Oczkowski A, Wigand C, Hanson A, Markham E, Miller KM, Johnson R. 2016. Nitrogen retention in salt marsh systems across nutrient-enrichment, elevation, and precipitation regimes: A multiple-stressor experiment. Estuaries and Coasts 39: 68–81. [Google Scholar]
- Orth RJ, et al. 2006. A global crisis for seagrass ecosystems. BioScience 56: 987–996. [Google Scholar]
- Paerl HW. 1995. Coastal eutrophication in relation to atmospheric nitrogen deposition: Current perspectives. Ophelia 41: 237–259. [Google Scholar]
- Paerl HW, Dennis RL, Whitall DR. 2002. Atmospheric deposition of nitrogen: Implications for nutrient over-enrichment of coastal waters. Estuaries 25: 677–693. [Google Scholar]
- Pascal P-Y, Fleeger JW.. 2013. Diverse dietary responses by saltmarsh consumers to chornic nutrient enrichment. Estuaries and Coasts 36: 1115–1124. [Google Scholar]
- Peierls BL, Caraco NF, Pace ML, Cole JJ. 1991. Human influence on river nitrogen. Nature 350: 386–387. [Google Scholar]
- Priest B. 2007. Effects of Elevation and Nutrient Availability on the Primary Production of Spartna alterniflora and the Stability of Southeastern Coastal Salt Marshes Relative to Sea Level Rise. Master's thesis, University of South Carolina, Columbia, South Carolina. [Google Scholar]
- Rivett MO, Buss SR, Morgan P, Smith JWN, Bemment CD. 2008. Nitrate attenuation in groundwater: A review of biogeochemical controlling processes. Water Research 42: 4215–4232. [DOI] [PubMed] [Google Scholar]
- Silliman BR, Zieman JC.. 2002. Top-down control of Spartina alterniflora production by periwinkle grazing in a Virgina salt marsh. Ecology 82: 2830–2845. [Google Scholar]
- Smil V. 2004. Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production. MIT Press. [Google Scholar]
- Smith DL, McLachlan J.. 1979. Nitrogen fixation, as determined by acetylene reduction, in two salt marshes of Minas Basin. Proceedings of the Nova Scotian Institute of Science 29: 381–392. [Google Scholar]
- Sousa AI, Lillebø AI, Caçador I, Pardal MA. 2008. Contribution of Spartina maritima to the reduction of eutrophication in estuarine systems. Environmental Pollution 156: 628–635. [DOI] [PubMed] [Google Scholar]
- Stewart WM, Dibb DW, Johnston AE, Smyth TJ. 2005. The contribution of commercial fertilizer nutrients to food production. Agronomy Journal 97: 1–6. [Google Scholar]
- Sullivan MJ, Currin CA.. 2002. Community structure and functional dynamics of benthic microalgae in salt marshes. Pages81–106 in Weinstein MP, Kreeger DA, eds. Concepts and Controversies in Tidal Marsh Ecology. Springer. [Google Scholar]
- Sundback K, Miles A.. 2000. Balance between denitrification and microalgal incorporation of nitrogen in microtidal sediments, NE Kattegat. Aquatic Microbial Ecology 22: 291–300. [Google Scholar]
- Tyler AC, Mastronicola TA, McGlathery KJ. 2003. Nitrogen fixation and nitrogen limitation of primary production along a natural marsh chronosequence. Oecologia 136: 431–438. [DOI] [PubMed] [Google Scholar]
- Tobias CR, Cieri M, Peterson BJ, Deegan LA, Vallino J, Hughes J. 2003a. Processing watershed-derived nitrogen in a well-flushed New England estuary. Limology and Oceanography 48: 1766–1788. [Google Scholar]
- Tobias CR, Giblin AE, McClelland J, Tucker J, Peterson BJ. 2003b. Sediment DIN fluxes are preferentially recycling of benthic microalgal nitrogen in a shallow macrotidal estuary. Marine Ecology Progress Series 257: 25–36. [Google Scholar]
- Valiela I, Cole ML.. 2002. Comparative evidence that salt marshes and mangroves may protect seagrass meadows from land-derived nitrogen loads. Ecosystems 5: 92–102. [Google Scholar]
- Valiela I, Teal JM.. 1979. The nitrogen budget of a salt marsh ecosystem. Nature 280: 652–656. [Google Scholar]
- Valiela I, Teal JM, Sass WJ. 1975. Production and dynamics of salt marsh vegetation and the effects of experimental treatment with sewage sludge: Biomass, production and species composition. Journal of Applied Ecology 12: 973–981. [Google Scholar]
- Valiela I, Costa J, Foreman K, Teal JM, Howes B, Aubrey D. 1990. Transport of groundwater-borne nutrients and their effects on coastal waters. Biogeochemistry 10: 177–197. [Google Scholar]
- Valiela I, Cole ML, Mcclelland J, Hauxwell J, Cebrian J, Joye SB. 2002. Role of salt marshes as part of coastal landscapes. Pages23–36 in Weinstein MP, Kreeger DA, eds. Concepts and Controversies in Tidal Marsh Ecology. Springer. [Google Scholar]
- Van Zomeren CM, White JR, DeLaune RD. 2011. Fate of nitrate in vegetated brackish coastal marsh. Soil Science of America Journal 76: 1919–1927. [Google Scholar]
- Verhoeven JT, Arheimer B, Yin C, Hefting MM. 2006. Regional and global concerns over wetlands and water quality. Trends in Ecology and Evolution 21: 96–103. [DOI] [PubMed] [Google Scholar]
- Viechtbauer W. 2010. Conducting meta-analyses in R with the metaphor package. Journal of Statistical Software 36: 1–48. [Google Scholar]
- Ward BB, Arp DJ, Klotz MG. 2011. Nitrification. American Society for Microbiology Press. [Google Scholar]
- Weller DE, Jordan TE.. 2020. Inexpensive spot sampling provides unexpectedly effective indicators of watershed nitrogen status. Ecosphere 11: e03224. [Google Scholar]
- Wigand C, Thursby GB, McKinney RA, Santos AF. 2004. Response of Spartina patens to dissolved inorganic nutrient additions in the field. Journal of Coastal Research 45: 134–149. [Google Scholar]
- Wigand C, Watson EB, Martin R, Johnson DS, Warren RS, Hanson A, Davey E, Johnson R, Deegan LA. 2018. Discontinuities in soil strength contribute to destabilization of nutrient-enriched creeks. Ecosphere 9: e02329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang L, Xie X, Huang L, Wu Y. 2013. Effects of invasion of Spartina alterniflora and exogenous N deposition on N2O emissions in a coastal marsh. Ecological Engineering 58: 77–83. [Google Scholar]
- Zumft W. 1997. Cell biology and molecular basis of denitrification. Microbiology and Molecular Biology Reviews 61: 533–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.