Abstract
Genetic background is a key but sometimes overlooked factor that profoundly impacts disease susceptibility and presentation in both humans and disease models. Here we show that deficiency of KLOTHO protein, an important renal regulator of mineral homeostasis and a cofactor for FGF23, causes different phenotypes in 129S1/SvlmJ (129) and C57BL/6J (B6) mouse strains. The 129 strain is more severely affected, with decreased longevity, decreased body weight, and increased amounts of kidney calcification compared with B6 mice. Reciprocal F1 crosses of the strains also indicate a parentage effect on the Klotho phenotype with F1 KLOTHO-deficient progeny of B6 mothers and 129 fathers having more kidney calcification than progeny of 129 mothers and B6 fathers. Comparing and contrasting the genetic architecture leading to different phenotypes associated with specific inbred mouse strains may reveal previously unrecognized and important metabolic interactions affecting chronic kidney disease.
Keywords: 129S1/SvlmJ (129), C57Bl/6J (B6), KLOTHO, parent of origin, renal disease
INTRODUCTION
According to the United States Renal Data System, chronic kidney disease (CKD) was estimated to be prevalent in ∼30 million Americans as of 2017 (18). Both aging and CKD are major causes of cardiovascular disease involving the deposition of calcium on the walls of the arteries, a process called vascular calcification (9). Originally identified as an antiaging factor (7), the KLOTHO protein is a very important regulator of renal function and thus of vascular calcification and other age-associated conditions (7, 13, 18). KLOTHO is mainly expressed in the kidneys and, along with other hormones and proteins, controls blood mineral levels. Mice with homozygous Klotho mutations (Kl/Kl) exhibit CKD symptoms. These include decreased body weight, and increased levels of serum phosphate that promote calcium deposition in blood vessels including the renal vasculature (6, 7, 13).
KLOTHO-deficient mice are a useful model for studying vascular calcification and renal dysfunction. However, the impact of mouse strain on the Klotho mutant phenotype has been given little attention. Understanding phenotypic differences between strains may open new insights into the physiological impact of this key regulator. Mouse strain has been shown to significantly impact susceptibility to vascular calcification associated with diet (2, 11, 12). A genetic analysis of 100 inbred strains made hyperlipidemic by the forced expression of the human genes encoding apolipoprotein E-Leiden (APOE-Leiden) and cholesteryl ester transfer protein (CETP) revealed many common atherosclerosis risk factors in mice and humans (2). Most importantly, this study identified candidate genes for further study. As with diet, chronic kidney disease and aging have a complex genetic relationship with vascular calcification. Consequently, we have initiated an investigation into how the Klotho mutant phenotype is manifested in two widely used, but genetically divergent, inbred mouse strains, C57BL/6J (B6) and 129S1/SvlmJ (129).
The genetic background of one’s parents also impacts health into adulthood. “Parent-of-origin” effects have been implicated in many human maladies, including dementia, psychiatric disorders, cancer, obesity, and diabetes (8). Potential mechanisms include genomic imprinting, maternal physiology, environmental impacts during gestation, and postnatal nutrition and care (5). Unlike human studies that are limited to association, the ability to arrange reciprocal F1 crosses between strains enables a more rigorous analysis of parent-of-origin effects in rodents (e.g., 1, 3, 10). We have derived unique congenic strains with the Klotho mutation on the B6 and 129 backgrounds. Consequently, our study includes a parallel investigation into the impact of parentage on the Klotho mutant phenotype.
To quantify the impact of KLOTHO deficiency in each strain, we compared body weight and renal calcification in healthy control mice and in mice homozygous for the Klotho hypomorphic mutation (7). We further tested for parent-of-origin effects by creating reciprocal crosses of each parental strain. We hypothesized that strain and/or parentage could influence body weight at adulthood (∼6 wk of age) and the level of renal calcium.
MATERIALS AND METHODS
Mice.
All animals were handled in accordance with the Guidelines for Care and Use of Experimental Animals and approved by NJ Medical School Institutional Animal Care and Use committee (protocol #TR201800052).
The KLOTHO-deficient (Kl/Kl) mice were a generous gift from Dr. Makoto Kuro-o by way of Dr. Sylvia Christakos (Rutgers, New Jersey Medical School). The genetic background of the original KLOTHO mice was a mixture of C57BL/6J, FVB, and C3H/J strains. These mice were backcrossed to either C57Bl/6J (B6) or 129S1/SvlmJ (129) mice acquired from Jackson Laboratory. The Klotho allele-bearing 129 mice were fully congenic (greater than 10 backcrosses). The C57Bl/6J strain was backcrossed for six or seven generations. Single nucleotide polymorphism (SNP) genotyping (Taconic Biosciences, Rensselaer, NY) indicated that the B6 and 129 mice had reached 99.29% overall C57Bl/6J and 99.37% strain 129S1/SvlmJ genome contribution, respectively. After weaning, moistened mouse chow (LabDiet PicoLab Rodent Diet 20; Lab Supply, Fort Worth, TX) was placed on the bottom of the cage to ensure that the smaller and weaker KLOTHO-deficient mice had access to food. Heterozygote-by-heterozygote breeding produced both control mice and Klotho mutant homozygotes. Previously, we observed no statistically significant differences between mice bearing the heterozygous and wild-type Klotho genotypes in any measured parameter. Assessed parameters include body and organ weights, gene expression, and calcium levels (19). Because our analyses confirmed published data indicating that the Klotho mutation is fully recessive, heterozygous and wild-type samples are presented together as “healthy control” samples.
Genotyping.
Mice were genotyped by tail DNA and semiquantitative PCR as follows: an initial denaturation at 97°C for 5 min; followed by 28 cycles of denaturation at 94°C for 30 s; annealing at 55°C for 45 s; and extension at 72°C for 1 min; ending with a final cycle of 94°C for 1 min, 55°C for 1 min, and 72°C for 5 min. A common primer (TGGAGATTGGAAGTGGACG) and a wild type-specific primer (TTAAGGACTCCTGCATCTGC) amplified a 458 bp fragment from the wild-type Klotho allele. The common primer and a mutation-specific primer (CAAGGACCAGTTCATCATCG) amplified a 920 bp fragment from the Klotho mutant allele. Additional genotyping was performed by Transnetyx, Inc. (Cordova, TN).
Necropsy.
On the day of necropsy, mice were weighed and then killed via inhalation of an overdose of isoflurane. The heart was immediately perfused with phosphate-buffered saline (PBS), and kidneys were removed, rinsed in PBS, and flash-frozen in liquid nitrogen and stored at −80°C.
Biochemical assays.
Frozen tissues were ground in liquid nitrogen with a mortar and pestle. The frozen powder was solubilized in PBS, pH 7.4, with 0.16 mg/ml heparin by sonication for two 10 s intervals while on ice. The lysates were centrifuged at 15,000 g for 20 min. Calcium levels in the supernatants were determined with the Cayman Chemical Calcium Assay Kit (Ann Arbor, MI) and normalized for protein as measured with the Pierce BCA Protein Kit (ThermoFischer, Rockford. IL).
Statistical analysis and number of independent breeding pairs.
All statistical analyses were performed with GraphPad Prism 8.0. The difference between means was analyzed by unpaired t tests. ANOVA was used where noted. Differences were considered significant if P < 0.05. Each experimental group included mice from between five and 14 breeding cages. Each breeding cage contained one male and two females. Thus, a minimum of five fathers and 10 females contributed to the data shown in each group. One-way ANOVA failed to identify any significant differences between breeding cages within any experimental group, with one exception. One of 13 breeding cages that contributed body weight to the female Klotho homozygote data in Fig. 3A contained outlier mice. Calcium values for this cage did not differ significantly and did not alter the main findings.
Fig. 3.
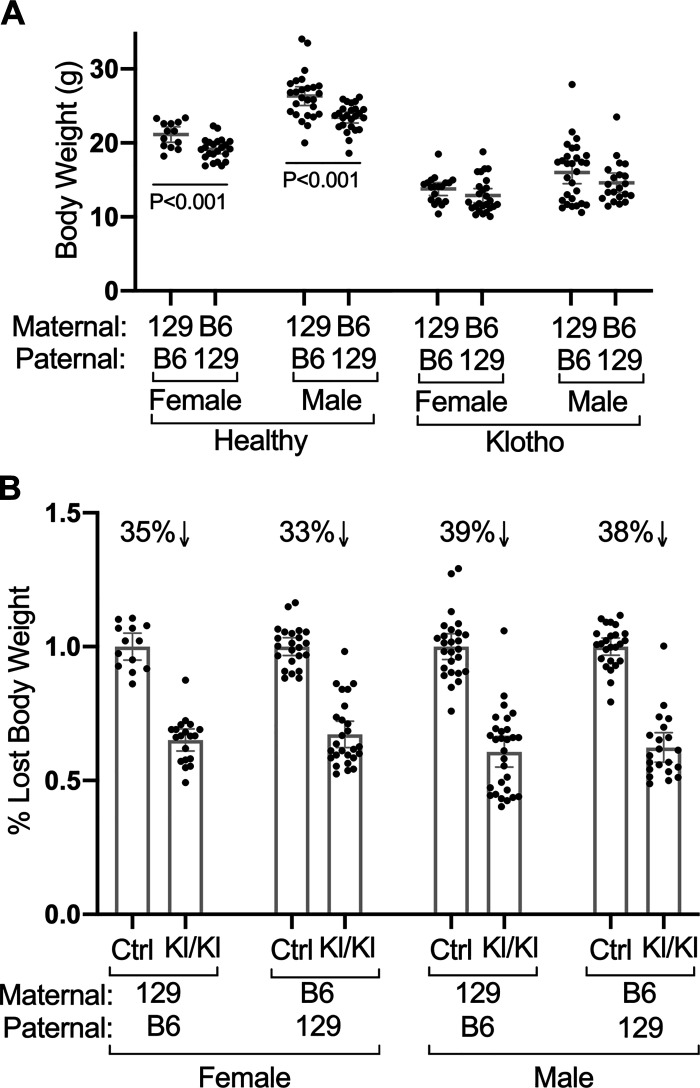
Reciprocal crosses indicate that parentage affects body weights in healthy control but not KLOTHO-deficient mice. A: weights for mice of indicated sexes, genotypes, and parental strains are shown. Female (n = 23) and male (n = 25) healthy F1 hybrids with B6 mothers and 129 fathers (B6/129F1) weigh significantly less than healthy F1 hybrids with 129 mothers and B6 fathers (n = 13 females and 26 males). In contrast, parentage did not significantly affect the body weight of diseased KLOTHO-deficient mice (129/B6F1 Kl/Kl n = 19 females and 29 males, B6/129F1 Kl/Kl n = 26 females and 21 males). B: relative to control healthy mice, mice with KLOTHO deficiency but different parentage lost similar amounts of body weight. Mean values ± 95% confidence interval are shown.
RESULTS
Impact of strain on Klotho deficiency phenotypes.
Genetic background influences susceptibility to disease in humans, and disease progression and severity often vary between strains of mice used as animal models of human disease. Comparing strains is crucial to identifying strain-specific results. We assessed KLOTHO deficiency phenotypes in two widely used inbred strains of mice: 129S1/SvlmJ (129) and C57BL/6J (B6). We compared body weights (Fig. 1) and kidney calcium content (Fig. 2) in mice with Klotho mutation homozygosity (Kl/Kl) and control mice of both strains.
Fig. 1.
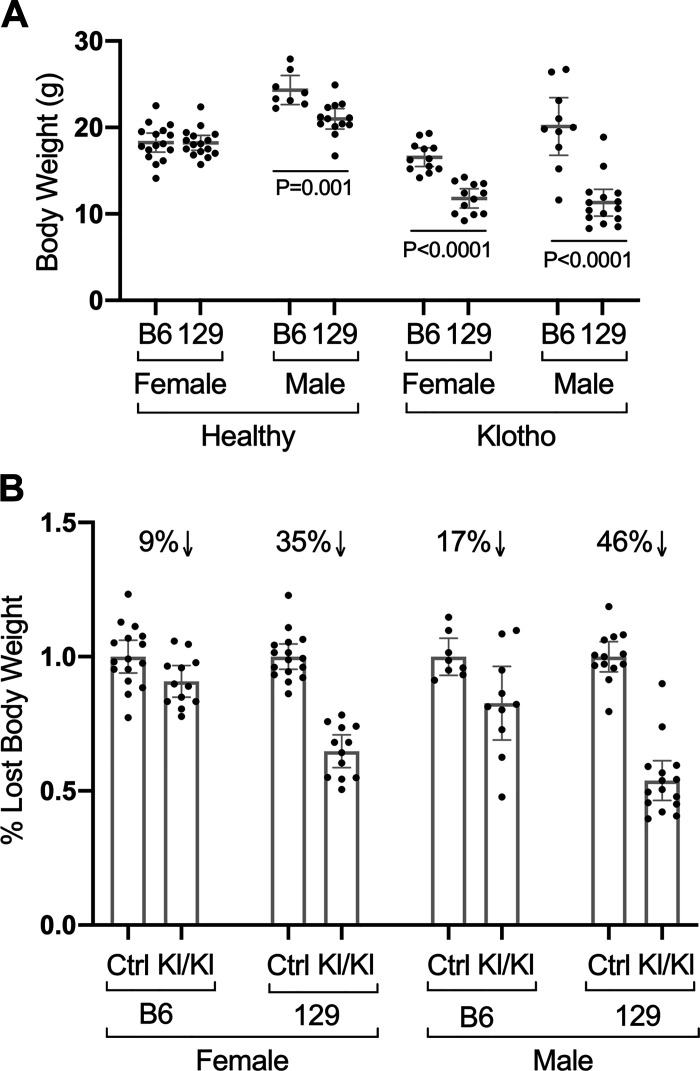
Strain affects adult body weight in healthy control (Ctrl) and KLOTHO-deficient (Kl/Kl) mice. A: weights for mice of the indicated strains (C57Bl/6J “B6” or strain 129S1/SvlmJ “129”), sexes and genotypes. Healthy B6 males (n = 8) weighed significantly more than healthy 129 males (n = 13). Diseased B6 Klotho homozygotes of both sexes (n = 13 females and 10 males) weighed more than 129 Klotho homozygotes (n = 12 females and 15 males). B: relative to control healthy mice, 129 mice with KLOTHO deficiency lost more body weight than B6 KLOTHO-deficient mice. Mean values ± 95% confidence interval are shown.
Fig. 2.
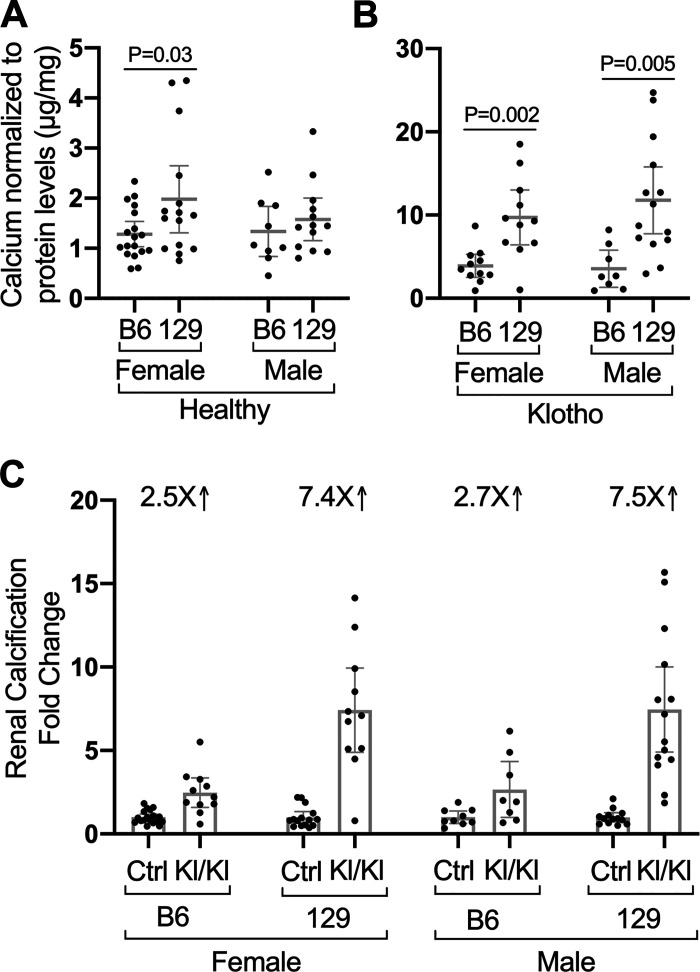
Strain affects the level of disease-associated calcification in kidneys from KLOTHO-deficient (Kl/Kl) mice. A, B: calcium levels normalized to protein levels in kidneys from mice of the indicated strains, sexes, and genotypes are shown. Diseased Klotho mutant homozygotes of both sexes and strains have increased levels of renal calcium compared with controls. Note different y-axes (Ctrl B6 n = 18 females and 9 males, Ctrl 129 n = 15 females and 13 males). B: KLOTHO-deficient B6 female (n = 11) and male mice (n = 8) have less kidney calcification than 129 mice (n = 11 females and 14 males). C: relative to control healthy mice, 129 mice with KLOTHO deficiency develop more renal calcification than B6 mice. Mean values ± 95% confidence interval are shown.
The KLOTHO-deficient (Kl/Kl) mice on the 129 background were noticeably ill, with lethargy, kyphosis, abnormal gait, decreased appetite, and difficulty breathing. In contrast, the KLOTHO-deficient B6 mice maintained a relatively healthy phenotype as reported previously (14). Male control mice on the B6 background weighed significantly more than males on the 129 background (P = 0.001), whereas strain did not alter the weight of female control mice as seen by others (11, 15).In contrast, strain profoundly influenced body weight in both sexes of KLOTHO-deficient mice (P < 0.0001 for each sex, Fig. 1A). The percent decrease of body weight relative to control mice in Kl/Kl 129 females was more than three times greater than that in B6 females (35 vs. 9%, respectively; Fig. 1B). In males, the relative decrease in body weight in 129 Kl/Kl mice was more than twice that of B6 males (46 vs. 17%, respectively; Fig. 1B).
Strain did not greatly alter the low levels of calcium content present in kidneys from control mice (Fig. 2A). However, kidneys from all Kl/Kl mice had more than twice as much calcium content relative to healthy mice (Fig. 2B) (P < 0.0001 by ANOVA). Previous studies have shown that calcium content reflects markedly increased vascular calcification (6, 7). Whereas KLOTHO deficiency in female B6 Kl/Kl mice was associated with a 2.5-fold calcium increase over control mice, Kl/Kl females on the 129 background exhibited a 7.4-fold increase over controls. Similarly, calcium content is increased 2.7-fold in male B6 Kl/Kl mice but increased 7.5-fold in the 129 background (Fig. 2C). The greater loss of body weight (Fig. 1) and higher levels of calcification in the kidneys (Fig. 2) associated with KLOTHO deficiency in strain 129 background relative to mice in B6 background supports a major effect of strain on the Klotho mutant phenotype.
This comparison had one limitation. A younger necropsy age was necessary for Klotho homozygous 129 mice because of their severe illness. Consequently, the average age of the Klotho homozygote male and female mice on the 129 background was significantly younger than those on the B6 background (47.9 ± 1.4 days as compared with 53.5 ± 2.5 days, respectively). Within each background, the control healthy mice were euthanized at the same ages as the KLOTHO-deficient (Kl/Kl) mice. Despite their younger age, kidney calcium levels in Klotho homozygotes on the 129 background were significantly greater than those on the B6 background. Furthermore, age of necropsy did not show significant correlation with calcium levels in either strain or sex (data not shown). The same Klotho mutation therefore resulted in different phenotypes in the two strains of mice. Specifically, KLOTHO deficiency in 129 mice caused greater loss of body weight and higher levels of calcification in the kidneys, whereas the Kl/Kl B6 mice were more resistant to calcification and disease.
Impact of parentage on Klotho deficiency phenotypes.
Inbred mice greatly improve experimental reproducibility. However, the striking differences in the Klotho mutant phenotype of each strain illustrate the hazards of drawing experimental conclusions from a single strain. F1 reciprocal hybrids between inbred strains are also genetically uniform, but, in contrast to inbred mice, F1 hybrids are heterozygous at each locus and exhibit hybrid vigor. Although genetic uniformity would generally promote phenotypic uniformity, the direction of each cross can influence phenotype. Consequently, we examined the effect of parentage on the phenotype of KLOTHO deficiency.
To expose possible parent-of-origin effects, we compared reciprocal crosses of 129 and B6 mice and measured their body weights as an indicator of robustness. Male and female healthy F1 hybrids with a 129 mother and a B6 father (129/B6F1) weighed significantly more than healthy B6/129F1 hybrids with a B6 mother and a 129 father (B6/129F1, Fig. 3A). As in inbred strains, Kl/Kl F1 hybrids weighed significantly less than the corresponding healthy F1 controls (compare Fig. 3A and 3B). However, the direction of the reciprocal cross failed to significantly affect the absolute weights of F1 Kl/Kl mice (Fig. 3A) or the percent weight loss (Fig. 3B). Thus, the impact of parent-of-origin on body weight appears to be overcome by the extreme impact of KLOTHO deficiency.
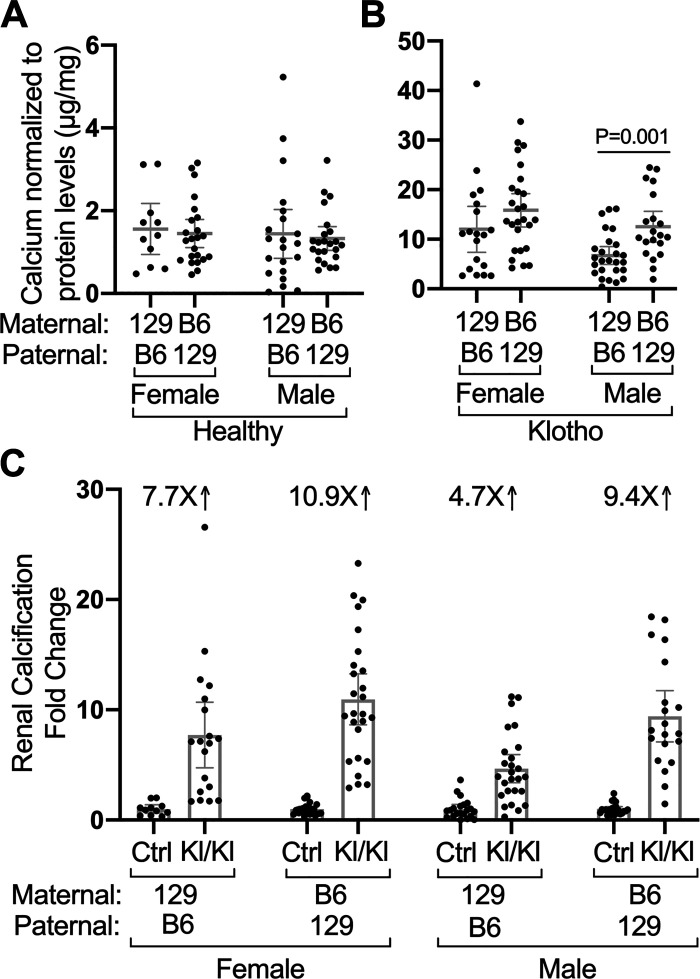
Although the weights of healthy B6/129F1 mice differed significantly from 129/B6F1 mice (Fig. 3A), kidney calcium content was very similar in the control mice (Fig. 4A). As in diseased inbred strains, the kidneys of Kl/Kl F1 hybrids were extensively calcified relative to control mice (Fig. 4, B and C). However, the B6/129F1 Kl/Kl male kidneys were significantly more calcified than the 129/B6F1 Kl/Kl male kidneys (P = 0.001). Females exhibited similar, but not significant, trend to greater calcification in the B6/129F1 hybrids. In summary, parentage affects body weight and KLOTHO deficiency associated calcification, but less profoundly than strain.
Fig. 4.
Reciprocal crosses indicate that parentage affects the level of disease-associated calcification in kidneys from KLOTHO-deficient (Kl/Kl) mice. A, B: calcium normalized to protein levels in kidneys from mice of indicated sexes, genotypes, and parental strains are shown. Diseased Klotho mutant homozygotes of all sexes and parentage (129/B6F1 Kl/Kl n = 19 female and 26 males, B6/129F1 Kl/Kl n = 26 females and 20 males) have increased levels of renal calcium compared with controls. Note different y-axes. (129/B6F1 Ctrl n = 11 females and 21 males, B6/129F1 Ctrl n = 23 females and 23 males). C: relative to control healthy mice, F1 hybrids with B6 mothers and 129S1 fathers develop more renal calcification than F1 hybrids with 129S1 mothers and B6 fathers. Mean values ± 95% confidence interval are shown.
DISCUSSION
Chronic kidney disease often develops over many years. Unfortunately, early diagnosis is rare. KLOTHO deficiency may be one of the earlier signs of renal failure, but circulating KLOTHO protein is challenging to measure (17). Understanding all the genetic factors that control the onset and pathological rate of this devastating illness may identify prognostic factors that make early therapy possible.
Genetic background is a key but sometimes overlooked factor that affects disease susceptibility and presentation in mouse disease models (4). The use of different mouse strains can lead to inconsistent and even conflicting results. However, understanding the impact of mouse strain may also provide clues to genetic modifiers of a pathological condition. In this study, we assessed the impact of KLOTHO deficiency in the widely used C57BL/6J (B6) and 129S1/SvlmJ (129) mouse strains. Mice deficient in KLOTHO on the B6 background appeared healthier for a longer life span, weighed more at necropsy, and had less renal calcification relative to KLOTHO-deficient mice on the 129 background (Figs. 1, 2). The more severe phenotype observed in 129 mice compared with B6 strain suggests that physiological differences impact disease progression. Indeed, bone, renal, and intestinal parameters including Ca absorption have been shown to differ in these strains (16). The increased severity observed in strain 129 is the reverse of that observed in hyperlipidemic models of atherosclerosis. B6 mice fed a high-fat diet with or without mutations that alter fat and cholesterol metabolism exhibited greater aortic calcification relative to strain 129 mice (2, 11). These two studies were large studies designed to identify genetic modifiers of atherosclerosis. Our findings suggest that the gene variants that modify vascular calcification in the context of kidney disease may differ from modifiers of high fat-associated disease.
Phenotypic differences associated with each inbred strain can be most simply explained by allelic variation of genes that directly or indirectly influence calcification. Disease susceptibility and progression are also affected by parent-of-origin effects, whereby the strain of the mother or father differently influences the phenotype of F1 hybrid offspring. These phenotypic differences include genomic imprinting whereby only one parental allele is expressed, maternal effects on gestational environment and/or postnatal maternal care and feeding, and mitochondrial or sex chromosome contributions (5, 8). By making reciprocal crosses between B6 and strain 129 parents, we showed that the renal calcification associated with KLOTHO deficiency (Fig. 4), but not body weight (Fig. 3), was influenced by parent of origin. Specifically, having a B6 mother and a strain 129 father increased renal calcification.
Translating results obtained at the bench to the bedside is extremely difficult. The genetic heterogeneity of human populations is one complicating factor. Mice offer the most experimentally tractable mammalian genetics, but an incomplete understanding of the impact of allelic heterogeneity on complex disease is another barrier to translating basic science to the clinic. Several large studies have described how genetic background impacts lipid dysregulation and atherosclerotic presentation in mice (2, 11). Our study is the first to demonstrate that the genetic background of both individual mice and that of their parents influences the pathological calcification associated with renal failure. Our study is only the first step toward understanding the genetic architecture that influences how renal dysregulation leads to pathological calcification. A comprehensive systems analysis in 100 mouse strains of genetic susceptibility to atherosclerosis in hyperlipidemic conditions has been performed (2, 11). A similar data set that evaluates the impact of genetic background on atherosclerosis and renal failure would help identify novel factors and candidate genes. Renal disease and the resulting cardiovascular pathologies are predicted to be a growing public health problem. Both hyperlipidemia and renal failure result in similar vascular pathology. Comparing and contrasting genetic similarities and differences in models of these diseases may reveal therapeutic approaches best suited for each situation.
GRANTS
Funding was provided by National Institutes of Health Grants (R01HL114751 and R56AG050762) to M. B. Rogers.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.E.G. and M.B.R. conceived and designed research; J.S.S. and D.E.G. performed experiments; J.S.S., D.E.G., and M.B.R. analyzed data; J.S.S., D.E.G., and M.B.R. interpreted results of experiments; J.S.S., D.E.G., and M.B.R. prepared figures; J.S.S. and D.E.G. drafted manuscript; J.S.S., D.E.G., and M.B.R. edited and revised manuscript; D.E.G. and M.B.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We warmly thank Youhua Zhu, Amy Song, and Khushboo Sahay for diligent technical assistance and Yue Wang for data analysis suggestions.
REFERENCES
- 1.Barrick CJ, Dong A, Waikel R, Corn D, Yang F, Threadgill DW, Smyth SS. Parent-of-origin effects on cardiac response to pressure overload in mice. Am J Physiol Heart Circ Physiol 297: H1003–H1009, 2009. doi: 10.1152/ajpheart.00896.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett BJ, Davis RC, Civelek M, Orozco L, Wu J, Qi H, Pan C, Packard RR, Eskin E, Yan M, Kirchgessner T, Wang Z, Li X, Gregory JC, Hazen SL, Gargalovic PS, Lusis AJ. Genetic Architecture of Atherosclerosis in Mice: A Systems Genetics Analysis of Common Inbred Strains. PLoS Genet 11: e1005711, 2015. [Erratum in PLoS Genet 12: e1005913, 2016] doi: 10.1371/journal.pgen.1005711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boubred F, Daniel L, Buffat C, Feuerstein JM, Tsimaratos M, Oliver C, Dignat-George F, Lelièvre-Pégorier M, Simeoni U. Early postnatal overfeeding induces early chronic renal dysfunction in adult male rats. Am J Physiol Renal Physiol 297: F943–F951, 2009. doi: 10.1152/ajprenal.90704.2008. [DOI] [PubMed] [Google Scholar]
- 4.Brayton CF, Treuting PM, Ward JM. Pathobiology of aging mice and GEM: background strains and experimental design. Vet Pathol 49: 85–105, 2012. doi: 10.1177/0300985811430696. [DOI] [PubMed] [Google Scholar]
- 5.Hager R, Cheverud JM, Wolf JB. Maternal effects as the cause of parent-of-origin effects that mimic genomic imprinting. Genetics 178: 1755–1762, 2008. doi: 10.1534/genetics.107.080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu MC, Shi M, Zhang J, Quiñones H, Griffith C, Kuro-o M, Moe OW. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 22: 124–136, 2011. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51, 1997. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 8.Lawson HA, Cheverud JM, Wolf JB. Genomic imprinting and parent-of-origin effects on complex traits. Nat Rev Genet 14: 609–617, 2013. doi: 10.1038/nrg3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neven E, D’Haese PC. Vascular calcification in chronic renal failure: what have we learned from animal studies? Circ Res 108: 249–264, 2011. doi: 10.1161/CIRCRESAHA.110.225904. [DOI] [PubMed] [Google Scholar]
- 10.Oreper D, Schoenrock SA, McMullan R, Ervin R, Farrington J, Miller DR, de Villena FP, Valdar W, Tarantino LM. Reciprocal F1 Hybrids of Two Inbred Mouse Strains Reveal Parent-of-Origin and Perinatal Diet Effects on Behavior and Expression. G3 (Bethesda) 8: 3447–3468, 2018. doi: 10.1534/g3.118.200135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paigen B, Bouchard G, Carey MC. Diet effects on gallstone formation and the assessment of liver morphology, plasma lipids, and atherosclerosis in 44 inbred strains of mice on high-fat atherogenic diet (not under pathogen-free conditions). The Jackson Laboratory; https://phenome.jax.org. [21 Jan., 2016]. [Google Scholar]
- 12.Paigen B, Morrow A, Brandon C, Mitchell D, Holmes P. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis 57: 65–73, 1985. doi: 10.1016/0021-9150(85)90138-8. [DOI] [PubMed] [Google Scholar]
- 13.Pescatore LA, Gamarra LF, Liberman M. Multifaceted Mechanisms of Vascular Calcification in Aging. Arterioscler Thromb Vasc Biol 39: 1307–1316, 2019. doi: 10.1161/ATVBAHA.118.311576. [DOI] [PubMed] [Google Scholar]
- 14.Phelps M, Pettan-Brewer C, Ladiges W, Yablonka-Reuveni Z. Decline in muscle strength and running endurance in klotho deficient C57BL/6 mice. Biogerontology 14: 729–739, 2013. doi: 10.1007/s10522-013-9447-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Philip VM, Sokoloff G, Ackert-Bicknell CL, Striz M, Branstetter L, Beckmann MA, Spence JS, Jackson BL, Galloway LD, Barker P, Wymore AM, Hunsicker PR, Durtschi DC, Shaw GS, Shinpock S, Manly KF, Miller DR, Donohue KD, Culiat CT, Churchill GA, Lariviere WR, Palmer AA, O’Hara BF, Voy BH, Chesler EJ. Genetic analysis in the Collaborative Cross breeding population. Genome Res 21: 1223–1238, 2011. doi: 10.1101/gr.113886.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Replogle RA, Li Q, Wang L, Zhang M, Fleet JC. Gene-by-diet interactions influence calcium absorption and bone density in mice. J Bone Miner Res 29: 657–665, 2014. doi: 10.1002/jbmr.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez-Niño MD, Fernandez-Fernandez B, Ortiz A. Klotho, the elusive kidney-derived anti-ageing factor. Clin Kidney J 13: 125–127, 2019. doi: 10.1093/ckj/sfz125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saran R, Robinson B, Abbott KC, Agodoa LYC, Bhave N, Bragg-Gresham J, Balkrishnan R, Dietrich X, Eckard A, Eggers PW, Gaipov A, Gillen D, Gipson D, Hailpern SM, Hall YN, Han Y, He K, Herman W, Heung M, Hirth RA, Hutton D, Jacobsen SJ, Jin Y, Kalantar-Zadeh K, Kapke A, Kovesdy CP, Lavallee D, Leslie J, McCullough K, Modi Z, Molnar MZ, Montez-Rath M, Moradi H, Morgenstern H, Mukhopadhyay P, Nallamothu B, Nguyen DV, Norris KC, O’Hare AM, Obi Y, Park C, Pearson J, Pisoni R, Potukuchi PK, Rao P, Repeck K, Rhee CM, Schrager J, Schaubel DE, Selewski DT, Shaw SF, Shi JM, Shieu M, Sim JJ, Soohoo M, Steffick D, Streja E, Sumida K, Tamura MK, Tilea A, Tong L, Wang D, Wang M, Woodside KJ, Xin X, Yin M, You AS, Zhou H, Shahinian V. US Renal Data System 2017 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 71, Suppl 1: A7, 2018. [Erratum in Am J Kidney Dis 71: 501, 2018] doi: 10.1053/j.ajkd.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang Y, Shah TA, Yurkow EJ, Rogers MB. MicroRNA profiles in calcified and healthy aorta: therapeutic impact of miR-145 and miR-378. Physiol Genomics, in press, 2020. doi: 10.1152/physiolgenomics.00074.2020. [DOI] [PubMed]