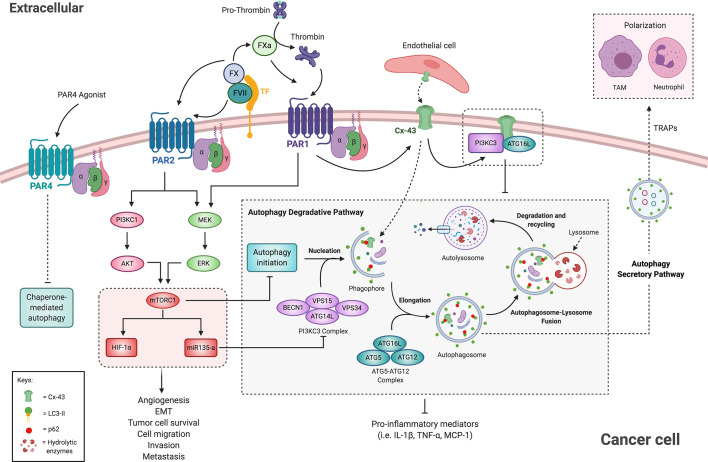
Figure 5.
Crosstalk of the coagulation cascade and autophagy pathway in cancer cells. The activation of PAR1 and PAR2 by coagulation factors suppresses the autophagic pathway in an mTORC1-dependent manner. mTORC1 upregulates HIF1-α and miR135, both implicated in cancer and in autophagy regulation. PAR1 activation leads to Cx-43 up-regulation, which may impair autophagy by sequestering ATG16L and the PI3KC3 complex to the membrane. In turn, the autophagic pathway mediates PAR1 degradation. Additionally, PAR4 selective stimulation downregulates proteins associated with chaperone-mediated autophagy. Therefore, autophagy suppression by PARs activation leads to increased pro-inflammatory microenvironment and enhanced cancer progression and metastasis. Moreover, the autophagy secretory pathway participates in TRAPs release, which suppress anti-tumor immune response and thereby facilitate tumor progression. See the text for additional information. Cx-43, Connexin 43; EMT, Epithelial to Mesenchymal Transition; ERK, Extracellular-signal Regulated Kinase; FVII, Factor VII; FX, Factor X; FXa, activated FX; HIF1-α, Hypoxia-Inducible Factor-1; LC3, microtubule-associated protein 1A/1B-light chain 3; MEK, mitogen-activated protein kinase; MCP1, monocyte chemoattractant protein 1; Monocyte mTORC1, mammalian target of rapamycin complex 1; PAR, protease-activated receptor; PI3K, phosphatidylinositol 3-kinase; TAM, tumor-associated macrophage; TF, tissue factor; TRAPs, tumor cell-released autophagosomes.