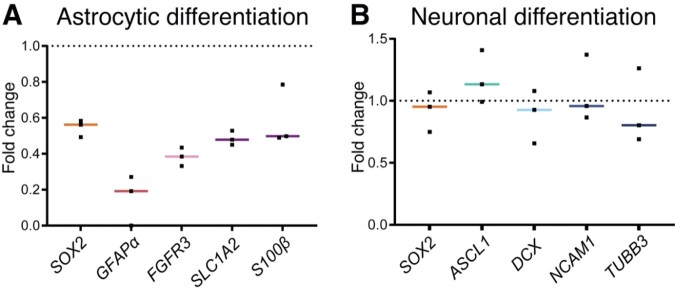
Figure 7.
Ventricular CSF exposure (7 days) primes ihNSCs to differentiate into neuronal over astrocytic lineage upon CSF withdrawal. (A) CSF-treated ihNSCs were differentiated according to astrocytic differentiation protocol upon CSF withdrawal (culture replicates, n = 3). Per-culture replicate, ihNSCs were untreated, respectively, pre-treated with pooled CSF of healthy controls. After 7 days, mRNA levels of SOX2 (Mann–Whitney U test, U = 0, P = 0.1000), GFAPα (Mann–Whitney U test, U = 0, P = 0.2000), FGFR3 (Mann–Whitney U test, U = 0, P = 0.1000), SLC1A2 (Mann–Whitney U test, U = 0, P = 0.1000), S100β (Mann–Whitney U test, U = 1, P = 0.2000) were measured. (B) Seven days later, ihNSCs were allowed to differentiate according to neuronal differentiation protocol (culture replicates, n = 3), after pre-treatment with pooled CSF, mRNA levels of SOX2 (Mann–Whitney U test, U = 3, P = 0.7000), ASCL1 (Mann–Whitney U test, U = 1, P = 0.2000), DCX (Mann–Whitney U test, U = 3, P = 0.7000), NCAM1 (Mann–Whitney U test, U = 4, P > 0.9999) and TUBB3 (Mann–Whitney U test, U = 4, P > 0.9999) were measured. Per-culture replicate, ihNSCs were untreated, respectively, pre-treated with pooled CSF of healthy controls. Actin, AluS and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as reference genes. Data are expressed as median fold change of relative mRNA expression of pre-treated ihNSCs over relative expression of untreated ihNSCs (dotted line); Sidak corrected P value = 0.0102.