Abstract
Temperamental cattle tend to yield carcasses of poorer quality, and Brahman cattle are reportedly more temperamental than non-indicus cattle breeds. A potential link between temperament and product quality may be mitochondrial activity. We hypothesized that mitochondrial measures would be greater in temperamental compared with calm heifers and that the relationships between temperament and mitochondria would persist as heifers age. Serum cortisol and skeletal muscle (longissimus thoracis [LT] and trapezius [TRAP]) mitochondrial profiles and antioxidant activities were quantified from the same calm (n = 6) and temperamental (n = 6) Brahman heifers at 8, 12, and 18 mo of age. Data were analyzed using a mixed model ANOVA in SAS (9.4) with repeated measures. Serum cortisol was greater in temperamental compared with calm heifers throughout the study (P = 0.02). Mitochondrial volume density (citrate synthase [CS] activity) increased over time (P < 0.0001) but was similar between temperament and muscle groups. Mitochondrial function (cytochrome c oxidase activity) was greatest in the temperamental LT at 8 mo of age (P ≤ 0.0006), greatest in the temperamental TRAP at 18 mo of age (P ≤ 0.003), and did not differ by temperament at 12 mo of age. Integrative (relative to tissue wet weight) mitochondrial oxidative phosphorylation capacity with complex I substrates (PCI), PCI plus complex II substrate (PCI+II), noncoupled electron transfer system capacity (ECI+II), and E with functional complex II only (ECII) were greater in the TRAP than LT for calm heifers at all ages (P ≤ 0.002), but were similar between muscle groups in temperamental heifers. Overall, calm heifers tended to have greater intrinsic (relative to CS activity) PCI and flux control of PCI+II (P ≤ 0.1) than temperamental heifers, indicating greater utilization of complex I paired with greater coupling efficiency in calm heifers. Within the LT, integrative PCI+II was greater (P = 0.05) and ECI+II tended to be greater (P = 0.06) in temperamental compared with calm heifers. From 8- to 18-mo old, glutathione peroxidase (GPx) activity decreased (P < 0.0001) and superoxide dismutase activity increased (P = 0.02), and both were similar between muscle groups. The activity of GPx was greater in temperamental compared with calm heifers at 8 (P = 0.004) but not at 12 or 18 mo of age. These results detail divergent skeletal muscle mitochondrial characteristics of live Brahman heifers according to temperament, which should be further investigated as a potential link between temperament and product quality.
Keywords: antioxidants, Brahman heifers, cortisol, mitochondrial capacity, muscle, temperament
Introduction
Recent work indicated that Brahman-influenced calves were significantly discounted in live animal prices compared with non-Brahman calves (Russell et al., 2016b). This economic penalty may be due to Brahman cattle being reportedly more temperamental than non-indicus cattle breeds and yielding carcasses of lesser quality (Cover et al., 1957; Fordyce et al., 1988; Kerth, 2013; Ponnampalam et al., 2017). In view of recent efforts focused on the identification of biomarkers to predict meat quality (Cassar-Malek and Picard, 2016), we propose that skeletal muscle mitochondrial profiles in live animals may be related to temperament, which may serve as the link between temperament and product quality.
Mitochondria are vitally important organelles that are referred to as the powerhouse of the cell because they are a significant source of energy (Kühlbrandt, 2015). Mitochondria not only play pivotal regulatory energetic roles that determine cell growth, metabolism, stress responses, and even cell death (Bratic and Larsson, 2013) but are also the primary source of reactive oxygen species (ROS) production during elevated respiration, which is often concomitant with chronic stress (Brooks et al., 2005). Temperamental cattle have elevated circulating concentrations of the stress hormone and cortisol and exhibit an endophenotype of chronic stress (Curley et al., 2006). Therefore, temperamental cattle may be at particular risk for ROS-induced cell dysfunction or death if antioxidant mechanisms are insufficient to combat excess ROS production (Peternelj and Coombes, 2011).
The objectives of this initial, longitudinal study were to determine relationships between temperament, age, and skeletal muscle mitochondrial capacity and antioxidant enzyme activities in Brahman heifers. We hypothesized that temperamental heifers would have greater mitochondrial function and capacity and antioxidant capacity (related to greater ROS production) than calm heifers. We further hypothesized that these temperament-related differences would persist as the heifers aged.
Materials and Methods
All care, procedures, and handling of animals were reviewed and approved by the Texas A&M AgriLife Research Agriculture Animal Care and Use Committee.
Cattle
Cattle were maintained on Coastal bermudagrass or ryegrass pastures at the Texas A&M AgriLife Research and Extension Center in Overton, TX. After recovery from the stress of weaning, the 6 calmest and 6 most temperamental Brahman heifers from a population of 50 individuals were selected for the study. Mean (±SEM) age and weight at weaning was 6.6 0.1 mo and 199.0 0.1 kg, respectively. Temperament was determined by exit velocity and pen score as previously described (Curley et al., 2006). Throughout the study, the administration of vaccines and parasiticides was uniform among all heifers and consistent with current best management practices.
Sample collection
Blood and muscle samples were collected from all heifers at 8, 12, and 18 mo of age. To facilitate sample collection and processing, heifers were randomly divided into four groups of three heifers each with at least one heifer of each temperament represented in each of the four groups. At each collection period (8, 12, and 18 mo), samples were collected from one group of three heifers per day over four consecutive days, beginning at 0800 hours each day.
At each sampling period, approximately 10 mL of blood was collected from each heifer by venipuncture of the jugular vein into evacuated tubes (Vacutainer; Becton, Dickson and Co., Franklin Lakes, NJ) free of anticoagulant. For serum harvest, samples were allowed to clot on ice for approximately 6 h and then were centrifuged at 1,600 g with the supernatant aliquoted and replicates stored at −80 °C until analysis of cortisol concentration.
Tissue samples were collected from the longissimus thoracis (LT) and the cervical trapezius (TRAP) muscles to compare a postural muscle of high economic value to a semi-locomotory muscle of low economic value, respectively. Collection procedures were followed as previously described (Li et al., 2016). The site of collection for the TRAP muscle was in the center of a triangular shape created by tracing imaginary lines along the jugular groove, shoulder, and spinal column in the neck. The site of collection for the LT was adjacent to and along the thoracic vertebrae, between the 11th and 13th ribs, 2.54 cm lateral from the midline. For subsequent collections, we moved in a straight line of 1.27 cm toward the posterior of the animal from the prior biopsy site. Prior to muscle tissue collection, the area to be collected was clipped, cleaned with 7.5% betadine scrub, and rinsed with 70% ethanol. Local anesthetic (0.1 mL of Lidocaine; Sparhawk Laboratories, Inc., Lenexa, KS) was administered to the biopsy site subcutaneously to numb the dermal tissue. A 14-gauge needle was used to create the initial puncture through the skin. Tissue was collected using a 14-gauge tissue collection needle (SuperCore Semi-Automatic Biopsy Instrument, Argon Medical Devices, Frisco, TX) by insertion of the biopsy needle to a standardized depth of 3 to 5 cm based on animal age (increased depth as animals grew) into the muscle at a 90° angle. Following collection, an aluminum aerosol bandage was applied to the collection site to prevent infection.
Approximately, 200 mg of tissue was collected from the LT and TRAP for each heifer at each sampling period. One aliquot of tissue was immediately placed in ice-cold biopsy preservation solution (BIOPS; Oroboros, Innsbruck, Austria; 10 mM Ca-EGTA buffer, 0.1μM free calcium, 20 mM imidazole, 20 mM taurine, 50 mM K-MES, 0.5 mM dithiothreitol, 6.56 mM MgCl2, 5.77 mM adenosine triphosphate (ATP), and 15 mM phosphocreatine; pH 7.1) and placed on ice or stored at 4 °C for same-day (within 24 h post-collection) analysis of oxidative capacity by high-resolution respirometry (HRR). The remaining three samples were placed in cryovials and immediately flash-frozen in liquid nitrogen for analysis of citrate synthase (CS), cytochrome c oxidase (COX), glutathione peroxidase (GPx), and superoxide dismutase (SOD) activities; samples were stored at −80 °C until analysis. Before enzyme analyses, frozen muscle tissue was cryopulverized into a fine powder using a liquid nitrogen-cooled cryopulverizer (Spectrum Bessman Tissue Pulverizer; Spectrum Laboratories, Inc., Rancho Dominguez, CA).
Serum cortisol
Serum cortisol concentrations were analyzed by radioimmunoassay as previously described (Curley et al., 2006). Samples were assayed in duplicate in a single assay with a coefficient of variation (CV) of 5%.
Muscle mitochondrial enzymes
Mitochondrial volume density and function of skeletal muscle samples were determined by analysis of CS and COX activities, respectively (Larsen et al., 2012; Meinild Lundby et al., 2018), as previously described (White et al., 2017). Each cryopulverized skeletal muscle tissue sample was sonicated (F60 Sonic Dismembrator, Fisher Scientific, Waltham, MA) in sucrose homogenization buffer (20 mM Tris, 40 mM KCl, 2 mM EGTA, and 250 mM sucrose, pH 7.4) with 0.05% detergent (n-Dodecyl β-d-maltoside; Sigma D4641) on ice and centrifuged at 11,000 g for 3 min. The supernatant was stored at −80 °C until analysis. Enzymatic activities were measured using a microplate reader (Synergy HT, BioTek Instruments, 237 Winooski, VT, USA) as previously described (Spinazzi et al., 2012; Li et al., 2016). A 40-fold dilution of the muscle homogenate was used for the analysis of CS and COX. Briefly, CS activity was determined measuring the linear rate of reaction of free CoA-SH with 5,5-dithio-bis-(2-nitrobenzoic acid) at 412 nm at 37 °C, and COX activity was determined by measuring the linear rate of oxidation of fully reduced cytochrome c (cyt c) at 550 nm (Spinazzi et al., 2012). Samples were analyzed in duplicate, with intra-assay CVs of 3.4% and 3.2% and inter-assay CVs of 3.1% and 4.8% for CS and COX activities, respectively.
Enzyme activities in muscle homogenates were normalized to total protein (integrative activity) determined using the Bradford Protein Assay Kit (Thermo Scientific, Rockford, IL). The activity of COX was further normalized to mitochondrial content (intrinsic activity) by dividing by CS activity as a marker of mitochondrial volume density in the sample (Larsen et al., 2012; Meinild Lundby et al., 2018).
High-resolution respirometry
Samples stored on ice in BIOPS were cleaned of fat and connective tissue and analyzed for mitochondrial capacity by HRR within 24 h of collection as previously described (Li et al., 2016; Latham et al., 2019). Oxygen flux and respiratory states were determined by HRR with the following substrate-uncoupler-inhibitor titration protocol as described by Latham et al. (2019): 1) pyruvate (5 mM) and malate (2 mM) to support electron flow through complex I (CI) of the electron transport system (ETS; LEAK respiration); 2) adenosine diphosphate (ADP; 2.5 mM) to stimulate respiration (OXPHOS; P); 3) cyt c (10 μM) to assess outer mitochondrial membrane integrity (samples with responses to cyt c greater than 15% were excluded); 4) glutamate (10 mM) as an additional CI substrate (PCI) and succinate (10 mM) to support convergent electron flow through complex II (CII) of the ETS (PCI+II); 5) uncoupler carbonyl cyanide 3-chlorophenylhydrazone (0.5 μM steps) to assess maximum noncoupled ETS capacity (ECI+II); 6) rotenone (0.5 µM), an inhibitor of complex I, to measure ETS capacity of complex II (ECII); 7) antimycin A (2.5 μM), an inhibitor of complex III, to measure residual oxygen flux independent of the ETS. Experiments were conducted at 37 °C in hyperoxic (200 to 500 µM O2) conditions. Respiratory state fluxes are presented on an integrative (per mg tissue wet weight) and intrinsic (per CS activity) basis. To convert CS activity from nmol · min−1 · mg protein−1 to pmol · s−1 · mg tissue−1 for intrinsic HRR calculations, total protein for each sample was converted from mg protein/mL to mg protein/mg tissue using a known homogenization dilution factor of 1 mg tissue to 0.04 mL buffer. Subsequently, appropriate conversion factors for nmol to pmol and min to sec were used to complete the calculation. The sample flux control ratio (FCR) for each complex was calculated by dividing the flux in each complex by the sample’s ECI+II flux.
Antioxidant enzymes
SOD and GPx activities were measured using commercially available kits (SOD: NWK-SOD02; GPx: NWK-GPX01; Northwest Life Science Specialties, LTC, Vancouver, WA). Cryopulverized tissue was homogenized in extraction buffer (0.1 M KH2PO4, 1 mM EDTA, pH 7.2) at 1,400 rpm for 15 min at 4 °C, sonicated on ice, and centrifuged at 11,000 g for 3 min at 0 °C. The supernatant was collected and stored at −80 °C until analysis. Forty-fold dilutions were used for both antioxidant assays. Samples for SOD were analyzed in triplicate with an intra-assay CV of 3.8% and inter-assay CV of 3.8%; samples for GPx were analyzed in duplicate with an intra-assay CV of 4.0% and inter-assay CV of 9.1%.
Statistical analyses
Data were analyzed using a repeated measures analysis of variance in SAS (Version 9.4, SAS Institute Inc., Cary, NC). Fixed effects included temperament, muscle, age, and all interactions. Data were tested for normality and log-transformed prior to analysis if not normally distributed. All data are expressed as least squares means ± SEM. Significance was declared at P 0.05, and trends were declared at 0.05 < P 0.10.
Results
Serum cortisol
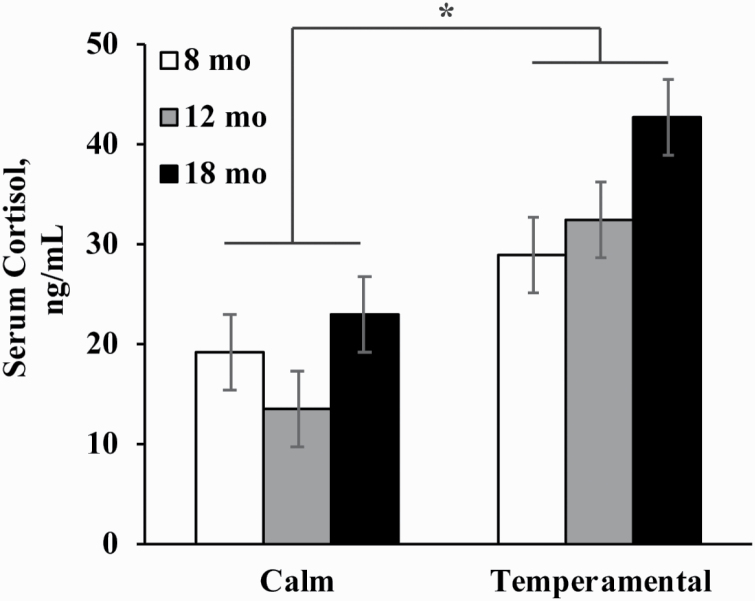
Temperamental heifers had greater serum cortisol than calm heifers through 18 mo of age (P = 0.02; Figure 1). There was a trend for an overall effect of age (P = 0.08), where serum cortisol was similar from 8 to 12 mo of age but increased from 12 to 18 mo of age (P = 0.04; Figure 1). There was no temperament × age interaction influencing serum cortisol concentration.
Figure 1.
Serum cortisol concentration in calm (n = 6) and temperamental (n = 6) Brahman heifers at 8, 12, and 18 mo of age. The overall effects of temperament (P = 0.02), age (P = 0.08), and temperament × age (P = 0.473) by repeated measures ANOVA. *Temperamental > Calm (P > 0.05).
Muscle mitochondrial enzymes
There was a trend for a muscle × age interaction (P = 0.06) but no effect of temperament on CS activity (Table 1). Overall, CS activity increased from 8 to 18 mo of age (P < 0.0001) but the temporal pattern of increase differed between the LT and TRAP. In the LT, CS activity was similar from 8 to 12 mo and increased from 12 to 18 mo of age (P = 0.0001). In contrast, CS activity increased from 8 to 12 mo (P < 0.0001) in the TRAP but plateaued from 12 to 18 mo of age (Table 1). The activity of CS was greater in the LT than the TRAP at 8 mo (P = 0.003) but was similar between muscle groups at 12 and 18 mo of age (Table 1).
Table 1.
CS and integrative (relative to mg protein) and intrinsic (relative to unit citrate synthase [U CS]) COX activities in the LT and TRAP muscles from calm (n = 6) and temperamental (Temp; n = 6) Brahman heifers at 8, 12, and 18 mo of age
| Variable | Temperament | Muscle | 8 mo | 12 mo | 18 mo | SEM | P-value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Temp | Musc | Age | Temp × Musc × Age | |||||||
| CS activity, nmol · min-1 · mg protein-1 | Calm | LT | 16.7ab | 26.2* | 38.2* | 2.2 | 0.760 | 0.039 | <0.0001 | 0.731 |
| TRAP | 12.1a | 28.0* | 31.4* | |||||||
| Temp | LT | 23.3b | 21.3 | 38.8* † | ||||||
| TRAP | 12.7a | 27.4* | 33.0* | |||||||
| Integrative COX activity, nmol · min-1 · mg protein-1 | Calm | LT | 20.3b | 7.4de* | 9.9x* | 2.1 | 0.522 | 0.174 | <0.0001 | 0.001 |
| TRAP | 11.0a | 13.3f | 12.6xy | |||||||
| Temp | LT | 31.3c | 4.6d* | 8.5x* | ||||||
| TRAP | 5.7a | 11.1ef | 18.0y* † | |||||||
| Intrinsic COX activity, nmol · min-1 · U CS-1 |
Calm | LT | 1.34c | 0.26d* | 0.27x* | 0.09 | 0.652 | 0.287 | <0.0001 | 0.167 |
| TRAP | 0.77b | 0.54e | 0.43xy* | |||||||
| Temp | LT | 1.37c | 0.19d* | 0.27x* | ||||||
| TRAP | 0.51a | 0.56e | 0.58y |
a–f,x,y Within variable and column, means with different letters differ (P < 0.05).
*Within row, mean differs from 8 mo (P < 0.05).
†Within row, mean differs from 12 mo (P < 0.05).
Integrative (relative to mg protein) COX activity was influenced by a three-way interaction between temperament, age, and muscle (P = 0.001; Table 1). Over time, COX activity decreased from 8 to 12 mo (P < 0.0001) but plateaued from 12 to 18 mo of age in calm and temperamental LT, remained unchanged through 18 mo of age in calm TRAP, and tended to increase from 8 to 12 mo (P = 0.1) and continued to increase from 12 to 18 mo of age in temperamental TRAP (P = 0.03; Table 1). At 8 mo of age, COX activity was greatest in the LT of temperamental heifers (P ≤ 0.0006), second greatest in the LT of calm heifers (P ≤ 0.003), and tended to be greater in calm compared with temperamental TRAP (P = 0.1; Table 1). At 12 mo of age, COX activity was greater in the TRAP than the LT (P = 0.005) but was similar between calm and temperamental heifers within each muscle group. By 18 mo of age, COX activity in temperamental TRAP was greater than calm and temperamental LT (P ≤ 0.003) and tended to be greater than calm TRAP (P = 0.08; Table 1).
Similar to CS activity, intrinsic (relative to CS activity) COX activity was influenced by a muscle × age interaction (P < 0.0001) but not by temperament (Table 1). Intrinsic COX activity was greater at 8 compared to 12 and 18 mo of age (P < 0.0001) in the LT. In contrast, intrinsic COX activity was unaffected by age in the TRAP (Table 1). At 8 mo of age, intrinsic COX activity was greater in the LT than the TRAP (P < 0.0001), whereas activity was greater in the TRAP than LT at both 12 and 18 mo of age (P 0.009; Table 1).
High-resolution respirometry
Integrative (relative to tissue wet weight) LEAK respiration increased at 18 mo of age (P < 0.0001) but was similar between muscles and unaffected by temperament (Table 2). Intrinsic (relative to unit CS) LEAK was greater at 8 compared with 12 and 18 mo of age (P < 0.0001); intrinsic LEAK also tended to be greater in the TRAP than the LT (P = 0.08; Table 2).
Table 2.
Integrative (relative to mg tissue) leak, PCI, PCI+II, ECI+II, and ECII in the LT and TRAP muscles from calm (n = 6) and temperamental (Temp; n = 6) Brahman heifers at 8, 12, and 18 mo of age
| O2 flux, pmol · sec-1 · mg tissue-1 |
Temperament | Muscle | 8 mo | 12 mo | 18 mo | SEM | P-value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Temp | Musc | Age | Temp × Musc × Age | |||||||
| LEAK | Calm | LT | 1.63 | 0.61 | 3.74† | 0.87 | 0.540 | 0.393 | <0.0001 | 0.866 |
| TRAP | 1.80 | 1.77 | 5.25*† | |||||||
| Temp | LT | 1.61 | 0.90 | 4.10† | ||||||
| TRAP | 1.32 | 0.15 | 3.88*† | |||||||
| PCI | Calm | LT | 21.2a | 10.4* | 21.3x† | 2.1 | 0.850 | 0.139 | <0.0001 | 0.365 |
| TRAP | 21.8a | 14.6* | 30.0y* † | |||||||
| Temp | LT | 28.9b | 10.5* | 22.3x* † | ||||||
| TRAP | 21.6a | 14.4* | 23.0x† | |||||||
| PCI+II | Calm | LT | 33.7a | 25.5* | 26.8x | 2.7 | 0.900 | 0.017 | <0.0001 | 0.306 |
| TRAP | 36.0ab | 32.3 | 44.9y* † | |||||||
| Temp | LT | 42.7b | 25.2* | 32.6x* | ||||||
| TRAP | 35.3ab | 27.9 | 34.3x | |||||||
| ECI+II | Calm | LT | 39.1 | 30.1d* | 32.3x | 2.9 | 0.750 | 0.0005 | 0.0002 | 0.798 |
| TRAP | 44.9 | 40.2e | 47.0z | |||||||
| Temp | LT | 46.8 | 30.8d* | 36.9xy* | ||||||
| TRAP | 45.6 | 35.9de | 42.1yz | |||||||
| ECII | Calm | LT | 21.9a | 21.8 | 16.6x | 1.9 | 0.688 | 0.024 | 0.070 | 0.325 |
| TRAP | 25.0ab | 23.8 | 26.8z | |||||||
| Temp | LT | 27.4b | 21.2* | 20.7xy* | ||||||
| TRAP | 24.3ab | 22.5 | 22.4yz |
a,b,d,e,x–zWithin variable and column, means with different letters differ (P < 0.05).
*Within row, mean differs from 8 mo (P < 0.05).
†Within row, mean differs from 12 mo (P < 0.05).
Integrative oxidative phosphorylation capacity (P) with complex I substrates (PCI) and with complex I+II substrates (well-coupled; PCI+II) were affected by interactions of muscle and temperament (P = 0.03) and muscle and age (P 0.02); PCI also showed a trend for an interaction of temperament and age (P = 0.09; Table 2). Within calm heifers, integrative PCI and PCI+II were greater in the TRAP than the LT (P 0.01) but were not different between muscles within temperamental heifers (Table 2). In both muscles and temperament groups, integrative PCI decreased from 8 to 12 mo (P < 0.0001) and increased from 12 to 18 mo of age (P < 0.0001; Table 2). In the LT and in temperamental heifers, PCI was similar at 18 compared with 8 mo of age, while PCI in the TRAP and in calm heifers was greater at 18 than 8 mo of age (P 0.05). This is primarily due to a marked increase in integrative PCI in the TRAP of calm heifers at 18 mo of age (P < 0.0001; Table 2). Calm heifers had greater integrative PCI+II than temperamental heifers in the TRAP (P = 0.03) but lesser PCI+II than temperamental heifers in the LT (P = 0.05; Table 2).
Similar to integrative PCI and PCI+II, both integrative ECI+II and E with complex II only (ECII) were greater in the TRAP than LT of calm (P 0.002) but were similar between muscles in temperamental heifers (Table 2). There was a trend for both measures to be greater in temperamental compared with calm LT (P 0.06; Table 2). Overall, ECI+II was greatest at 8 mo of age (P 0.05), least at 12 mo of age (P 0.02), and intermediate at 18 mo of age (Table 2). A trend for an effect of age (P = 0.07) suggested that integrative ECII tended to decrease from 8 to 12 mo of age (P = 0.08) and was lesser at 18 compared with 8 mo of age (P = 0.03; Table 2).
Intrinsic (relative to CS activity) PCI and PCI+II were affected by the interaction of muscle and age (P = 0.03; Table 3). Both measures decreased from 8 to 12 mo of age (P 0.04) and tended to increase from 12 to 18 mo of age (P 0.1) in the TRAP but were unaffected by age in the LT. As such, the TRAP had greater intrinsic PCI and PCI+II than the LT at 8 and 18 mo of age (P < 0.0001) but muscles were similar at 12 mo of age (Table 3). Calm heifers also tended to have greater intrinsic PCI than temperamental heifers (P = 0.1; Table 3).
Table 3.
Intrinsic (relative to CS activity) leak, PCI, PCI+II, ECI+II, and ECII in the LT and TRAP muscles from calm (n = 6) and temperamental (Temp; n = 6) Brahman heifers at 8, 12, and 18 mo of age
| O2 Flux, pmol · sec-1 · U CS-1 |
Temperament | Muscle | 8 mo | 12 mo | 18 mo | SEM | P-value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Temp | Musc | Age | Temp × Musc × Age | |||||||
| LEAK | Calm | LT | 1.46a | 0.07* | 0.17* | 0.27 | 0.939 | 0.083 | < 0.0001 | 0.513 |
| TRAP | 1.84ab | 0.10* | 0.32* | |||||||
| Temp | LT | 1.20a | 0.08* | 0.16* | ||||||
| TRAP | 2.32b | 0.10* | 0.18* | |||||||
| PCI | Calm | LT | 1.70a | 1.89 | 1.47x | 0.16 | 0.109 | < 0.0001 | 0.861 | 0.370 |
| TRAP | 2.60b | 2.18 | 2.88y | |||||||
| Temp | LT | 1.24a | 1.60 | 1.54x | ||||||
| TRAP | 2.66b | 1.87 | 2.40y | |||||||
| PCI+II | Calm | LT | 1.98a | 2.24 | 1.57x | 0.32 | 0.482 | < 0.0001 | 0.599 | 0.404 |
| TRAP | 3.22b | 2.70 | 3.27y | |||||||
| Temp | LT | 1.52a | 2.00 | 1.72x | ||||||
| TRAP | 3.53b | 2.45 | 2.96y | |||||||
| ECI+II | Calm | LT | 2.61 | 2.32de | 1.84 | 0.46 | 0.896 | 0.087 | 0.651 | 0.862 |
| TRAP | 2.75 | 3.59e | 2.10† | |||||||
| Temp | LT | 2.52 | 2.00d | 2.63 | ||||||
| TRAP | 2.66 | 2.67de | 2.93 | |||||||
| ECII | Calm | LT | 1.10ab | 1.59* | 0.86x† | 0.16 | 0.943 | < 0.0001 | 0.021 | 0.168 |
| TRAP | 1.54bc | 1.71 | 1.70y | |||||||
| Temp | LT | 0.84a | 1.36* | 0.96x | ||||||
| TRAP | 1.97c | 1.73 | 1.60y |
a–e,x,yWithin variable and column, means with different letters differ (P < 0.05).
*Within row, mean differs from 8 mo (P < 0.05).
†Within row, mean differs from 12 mo (P < 0.05).
Intrinsic ECI+II tended to be greater in the TRAP than LT at all ages (P = 0.09) and tended to be greater in temperamental than calm heifers at 18 mo of age (P = 0.08; Table 3). Intrinsic ECII, on the other hand, was greater at 12 than 8 or 18 mo of age (P 0.04) in the LT but was unaffected by age in the TRAP (Table 3). Intrinsic ECII was unaffected by temperament (Table 3).
Flux control ratios
FCRs signify the contribution of each complex to total E capacity. The FCR of LEAK (FCRL) was greater at 18 than 8 and 12 mo of age (P 0.0001) but was unaffected by temperament or muscle (Table 4). The FCR of complex I-supported P (FCRPCI) was greatest at 18 mo (P 0.0006), least at 12 mo (P < 0.0001), and intermediate at 8 mo of age (Table 4). At 8 mo of age, the LT tended to have a greater FCRPCI than TRAP (P = 0.07) but muscles were similar at 12 and 18 mo of age (Table 4). Overall, the coupling efficiency of the mitochondria supported by both complex I and II (FCRPCI+II) tended to be greater in calm than temperamental heifers (P = 0.09; Table 4). Additionally, the FCRPCI+II displayed a trend for a three-way interaction of age, muscle, and temperament (P = 0.1; Table 4). The FCRPCI+II was greater at 18 than 8 and 12 mo of age in the TRAP of calm heifers (P 0.04), and greater at 18 than 12 mo of age in the LT of temperamental heifers (P = 0.04), but was unaffected by age in the LT of calm and the TRAP of temperamental heifers. Further, the FCRPCI+II was greater in the LT than the TRAP in 8-mo-old calm heifers (P = 0.08), greater in calm LT than temperamental TRAP at 12 mo (P = 0.05), and greater in both the TRAP of calm heifers and the LT of temperamental heifers than the TRAP of temperamental heifers at 18 mo of age (P = 0.03; Table 4). The contribution of complex II-supported E to total E (FCRECII) was greater at 12 than 8 and 18 mo of age (P < 0.0001) but was unaffected by temperament and muscle (Table 4).
Table 4.
FCRL, FCRPCI, FCRPCI+II, and FCRECII in the LT and TRAP muscles from calm (n = 6) and temperamental (Temp; n = 6) Brahman heifers at 8, 12, and 18 mo of age
| FCR1 | Temperament | Muscle | 8 mo | 12 mo | 18 mo | SEM | P-value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Temp | Musc | Age | Temp × Musc × Age | |||||||
| FCRL | Calm | LT | 0.04 | 0.03 | 0.12* † | 0.02 | 0.599 | 0.998 | <0.0001 | 0.941 |
| TRAP | 0.03 | 0.05 | 0.11* † | |||||||
| Temp | LT | 0.04 | 0.03 | 0.10 | ||||||
| TRAP | 0.04 | 0.03 | 0.10† | |||||||
| FCRPCI | Calm | LT | 0.54 | 0.34* | 0.60† | 0.03 | 0.732 | 0.246 | <0.0001 | 0.625 |
| TRAP | 0.48 | 0.39* | 0.59* † | |||||||
| Temp | LT | 0.54 | 0.36* | 0.61† | ||||||
| TRAP | 0.49 | 0.38* | 0.54† | |||||||
| FCRPCI+II | Calm | LT | 0.86 | 0.85e | 0.86xy | 0.02 | 0.089 | 0.019 | 0.020 | 0.109 |
| TRAP | 0.80 | 0.82de | 0.89y* † | |||||||
| Temp | LT | 0.83 | 0.81de | 0.88y† | ||||||
| TRAP | 0.81 | 0.78d | 0.81x | |||||||
| FCRECII | Calm | LT | 0.58 | 0.73e* | 0.54† | 0.03 | 0.238 | 0.152 | <0.0001 | 0.562 |
| TRAP | 0.56 | 0.66de* | 0.56† | |||||||
| Temp | LT | 0.54 | 0.69de* | 0.57† | ||||||
| TRAP | 0.56 | 0.63d | 0.54† |
1Calculated as the variable of interest divided by the electron transfer capacity with complex I+II (ECI+II) of the sample.
d,e,x,yWithin variable and column, means with different letters differ (P < 0.05).
*Within row, mean differs from 8 mo (P < 0.05).
†Within row, mean differs from 12 mo (P < 0.05).
Antioxidant enzymes
GPx activity decreased as heifers aged from 8 to 18 mo (P < 0.0001; Table 5), while SOD activity increased during that time (P = 0.02; Table 5). The activity of GPx was greater in the TRAP than the LT at 8 and 12 mo of age (P 0.02) but was similar between muscle groups by 18 mo of age (Table 5). Additionally, GPx activity was greater in temperamental compared with calm heifers at 8 (P = 0.004) but not at 12 or 18 mo of age (Table 5). The activity of SOD was unaffected by temperament and muscle (Table 5).
Table 5.
GPx and SOD activities in the LT and TRAP muscles from calm (n = 6) and temperamental (Temp; n = 6) Brahman heifers at 8, 12, and 18 mo of age
| Variable | Temperament | Muscle | 8 mo | 12 mo | 18 mo | SEM | P-value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Temp | Musc | Age | Temp × Musc × Age | |||||||
| GPx Activity, mU/mg protein | Calm | LT | 7.2a | 8.4de | 4.5 | 2.3 | 0.136 | 0.0002 | < 0.0001 | 0.516 |
| TRAP | 16.4b | 10.1de* | 4.7* | |||||||
| Temp | LT | 14.6b | 6.2e* | 4.1* | ||||||
| TRAP | 24.6c | 14.8d* | 4.7* † | |||||||
| SOD Activity, U/mg protein | Calm | LT | 10.7 | 16.8 | 20.4 | 3.2 | 0.127 | 0.518 | 0.017 | 0.179 |
| TRAP | 12.0 | 17.1 | 16.4 | |||||||
| Temp | LT | 11.9 | 22.7* | 16.9 | ||||||
| TRAP | 18.1 | 17.6 | 25.8 |
a–eWithin variable and column, means with different letters differ (P < 0.05).
*Within row, mean differs from 8 mo (P < 0.05).
†Within row, mean differs from 12 mo (P < 0.05).
Discussion
The potential for an in vivo linkage of muscle mitochondrial measures and temperament of Brahman heifers was investigated in this study. Specific objectives were to determine the effects of temperament on serum cortisol and skeletal muscle mitochondrial profiles and antioxidant enzyme activities in Brahman heifers from 8 through 18 mo of age. The current study confirmed that temperamental heifers had elevated circulating cortisol persisting through 18 mo of age. Additionally, our novel in situ characterization of live animal skeletal muscle mitochondria demonstrated differences in complex-specific mitochondrial O2 consumption according to temperament that may have implications for future production outcomes.
Cortisol is known as a stress hormone because it is released following an acute stressor or injury to induce the “stress response,” a coordinated integration of various physiological systems and intermediary metabolism to enhance the likelihood of survival. For example, increased secretion of cortisol partitions energy resources by increasing lipolysis, proteolysis, and gluconeogenesis while decreasing glycogen and protein synthesis (Thau and Sharma, 2020). Cortisol enhances mitochondrial protein expression and function in various models [e.g., increased skeletal muscle expression and activity of COX in rats (Weber et al., 2002); increased OXPHOS proteins in trout skeletal muscle (Aedo et al., 2019)], which leads to an elevated basal metabolic rate. The effects of chronically elevated cortisol production on skeletal muscle mitochondrial function have not been investigated. However, increasing basal metabolism at the expense of lipid and protein synthesis would result in elevated nutrient requirements, decreasing efficiency of gain, and likely poorer quality meat production (e.g., decreased yield and decreased intramuscular lipid). This hypothesis is supported by reports that temperamental cattle have lesser body weight, average daily gain, and feed efficiency than calm cattle (Burdick et al., 2009; Haskell et al., 2014). However, greater expression of peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC1-α), the master regulator of mitochondrial biogenesis, was evident in the longissimus dorsi of low RFI (low feed:gain and high feed efficiency) Limousin Friesian heifers (Kelly et al., 2011), suggesting greater mitochondrial content in efficient production animals. The relationship between chronic cortisol exposure and mitochondria should be further investigated, especially as it relates to production efficiency in cattle.
Skeletal muscle mitochondrial volume density (measured as CS activity) has been shown to increase with percentage Brahman influence in a herd (Wright et al., 2018); however, temperament was not considered. Within Brahman heifers in the current study, mitochondrial volume density (CS activity) did not differ due to temperament but did increase with age. An increase in the oxidative phenotype of skeletal muscle during postnatal development has been documented in other species (Schiaffino and Reggiani, 2011; Kim et al., 2019). Additionally, in Saler and Limousin bulls, the percentage of type IIa (fast-twitch, oxidative) fibers increased at the expense of type IIx (fast-twitch, glycolytic) fibers in the semitendinosus muscle from 10 to 16 mo of age (Jurie et al., 1999). The current study is the first report of an age-related increase in mitochondrial volume density in skeletal muscle of young, growing Brahman heifers.
Mitochondria are highly dynamic and complex organelles that readily adapt to cellular energy requirements (Picard et al., 2011). In the inner membrane cristae, OXPHOS and electron transport take place via a set of five protein complexes that produce an electrochemical gradient, which drives the production of ATP (Bratic and Larsson, 2013). The complexes are as follows: complex I (nicotinamide adenine dinucleotide [NADH] dehydrogenase), complex II (succinate dehydrogenase), complex III (cyt c reductase), complex IV (COX), and complex V (ATP synthase; Kühlbrandt, 2015). Notably, complexes I and II are not codependent nor sequential and rely on different substrates for electron transport. Further, increased complex I activity is associated with elevated mitochondrial ROS production, while complex II serves as a source of reserve respiratory capacity that promotes cell survival during times of stress (St-Pierre et al., 2002; Pfleger et al., 2015). As such, animals that rely more heavily on complex I for energy production may experience greater levels of oxidative stress, which could decrease feed efficiency by shuttling energy away from tissue accretion to enhance antioxidant activity (Russell et al., 2016a).
The novel technique, HRR, allows for the investigation of muscle mitochondrial energetics in situ using permeabilized muscle fiber bundles, which retain normal morphology and intracellular mechanisms (Li et al., 2016). Ramos et al. (2020a) reported that integrative (relative to tissue wet weight) maximal OXPHOS capacity with complexes I and II (PCI+II) was greater in postmortem samples from Brahman compared with Angus steers. However, temperament was not considered in their analysis. Further, little is known about mitochondrial capacity measures in live cattle. Our current results revealed marked differences in OXPHOS and electron transfer capacities of mitochondria based on animal temperament. In both the LT and TRAP muscles, and at all three ages, calm heifers tended to have greater intrinsic complex-I supported OXPHOS (PCI) and FCRPCI+II than temperamental heifers. The FCRPCI+II serves as an indicator of the efficiency of mitochondrial coupling, where a value of 1 would indicate 100% of electrons being transferred are coupled with oxidative phosphorylation. One caveat is that we included LEAK respiration in the calculation for FCRPCI+II, as has been suggested by the MitoEAGLE Task Group (Gnaiger and MitoEAGLE Task Group, 2020). LEAK respiration is a measure of proton leak across the inner mitochondrial membrane independent of ATP synthase (complex V); thereby, O2 consumption due to proton leak does not result in ATP production. Proton leak occurs via a variety of mechanisms including, but not limited to, uncoupling proteins and is thought to assist in binding ROS to prevent oxidative stress (Jastroch et al., 2010; Divakaruni and Brand, 2011). In our current study, LEAK respiration did not differ by muscle or temperament group, and was relatively low, contributing less than 10% of maximal electron transfer capacity. Interestingly, complex I is often associated with ROS production during exercise and disease (Di Meo et al., 2019). Since calm heifers had greater intrinsic complex I-supported capacity, a greater coupling efficiency may offset excess ROS production.
As additional measures against ROS production, several antioxidants work together in a coordinated and complementary system in the body (Niki et al., 1995). Enzymatic defenses, such as SOD, convert superoxide radicals to less toxic hydrogen peroxide (Bratic and Larsson, 2013), while GPx is responsible for the detoxification of peroxides and protection of the cell (Borek et al., 1986; Ghazi Harsini et al., 2012). The current study is the first to demonstrate a change in muscle antioxidant defenses with age in Brahman heifers; SOD activity increased with age while GPx activity decreased. Further, 8-mo-old temperamental heifers had greater GPx activity than calm heifers. The elevated GPx activity in 8-mo-old temperamental heifers may be related to their elevated integrative mitochondrial function (COX activity) in the LT at that age. With mitochondrial respiration being a primary source of ROS production, antioxidant defenses may have been upregulated to combat the increased ROS load (Ji, 2008).
In addition to decreased coupling efficiency in temperamental heifers, intrinsic noncoupled electron transfer capacity (ECI+II) tended to be greater in temperamental compared with calm heifers at 18 mo of age. Excess ECI+II without increased PCI+II is an inefficient utilization of mitochondrial electron transfer because electrons are likely being either lost as heat or potentially contributing to ROS rather than being coupled to ATP production. The elevation of noncoupled, excess ECI+II at 18 mo of age in temperamental heifers exacerbates the poor coupling efficiency, which may provide a mechanism for the decreased feed efficiency of temperamental cattle. In support, low feed-efficient broilers had greater excess ETS capacity compared with high feed-efficient animals (Bottje et al., 2002). Additionally, heat-stressed broilers were shown to have suppressed mitochondrial complex I activity (Huang et al., 2015), reminiscent of temperamental heifers having lesser intrinsic PCI compared with calm heifers in the current study. It is probable that differences in mitochondrial efficiency contribute to poor feed efficiency in temperamental Brahman cattle. Production efficiency was not recorded in the current study but the relationship between mitochondria, temperament, and feed efficiency in cattle warrants further investigation.
In the current study, we chose to compare mitochondrial measures in the economically valuable LT to the TRAP, a muscle with low economic value. The LT is a heterogeneous muscle group, composed of approximately 60% oxidative fibers (types I and IIa) by proportion but only about 55% oxidative fibers by relative area in mature, male cattle (Kadim et al., 2009; Costa et al., 2017). Early literature indicated the TRAP muscle may be less glycolytic than the LT, but the same study showed similar COX activity between the TRAP and longissimus dorsi muscles (Talmant et al., 1986). In the current study, all integrative measures of mitochondrial capacity (PCI, PCI+II, ECI+II, and ECII) were greater in the TRAP than the LT in calm heifers but similar between muscle groups in temperamental heifers, especially as animals aged. Additionally, in the LT, integrative PCI+II was greater in temperamental compared with calm heifers, and integrative ECI+II also tended to be greater in temperamental heifers. Previous work has indicated that increased mitochondrial activity in muscle results in poorer postmortem conversion to tender meat due to resistance to a decline in pH and impaired calpain-1 protein degradation (Wright et al., 2018; Ramos et al., 2020b). These elevations in mitochondrial capacities in the LT of temperamental heifers specifically may be related to the poorer meat quality associated with temperamental animals (Fordyce et al., 1988; King et al., 2006; Burdick et al., 2009; Cafe et al., 2010). This should be further investigated in steers followed to slaughter.
Overall, we have ascertained divergent skeletal muscle mitochondrial characteristics of live animals, which are linked to temperament in Brahman heifers. Future research should include comparisons of live animal measures of mitochondrial function with product quality at harvest (shear force, L*, a*, b*, etc.) in animals of both Bos taurus and Bos indicus breeding. This information may assist in optimizing handling processes for both live animals and post-harvest beef products according to mitochondrial profiles.
Acknowledgments
We would like to thank D.A. Neuendorff, D.A. Law, and C.L. Wellman for technical expertise and assistance with sample collections. This project was supported in part by Multistate Hatch projects W-3112 and NC1184, Hatch projects TEX07704 and H-8004, and the Texas A&M University Department of Animal Science Mini-Grant program.
Glossary
Abbreviations
- ATP
adenosine triphosphate
- BIOPS
biopsy preservation solution
- 10 mM Ca-EGTA buffer
0.1mM free calcium, 20 mM imidazole, 20 mM taurine, 50 mM K-MES, 0.5 mM dithiothreitol, 6.56 mM MgCl2, 5.77 mM ATP, and 15 mM phosphocreatine, pH 7.1
- CI
complex I
- CII
complex II
- COX
cytochrome c oxidase
- CS
citrate synthase
- cyt c
cytochrome c
- ECI+II
maximal electron transfer capacity not coupled with oxidative phosphorylation (noncoupled)
- ECII
electron transfer capacity with functional complex II (complex I inhibited)
- ETS
electron transport system
- FCR
flux control ratio
- FCRECII
flux control ratio of electron transfer with functional CII
- FCRL
flux control ratio of LEAK
- FCRPCI
flux control ratio of coupled oxidative phosphorylation with complex I substrates
- FCRPCI+II
flux control ratio of well-coupled oxidative phosphorylation with complex I and II substrates
- GPx
glutathione peroxidase
- HRR
high-resolution respirometry
- LEAK
LEAK respiration; uncoupled consumption of O2 prior to adenosine diphosphate addition during high-resolution respirometry
- LT
longissimus thoracis muscle
- OXPHOS
oxidative phosphorylation
- P
oxidative phosphorylation capacity
- PCI
oxidative phosphorylation with CI substrates, pyruvate, malate, and glutamate
- PCI+II
well-coupled oxidative phosphorylation with CI substrates, pyruvate, malate, and glutamate, and complex II substrate, succinate
- PGC1-a
peroxisome proliferator-activated receptor gamma coactivator 1-a
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TRAP
trapezius muscle
Conflicts of interest statement
The authors declare that they have no conflicts of interest.
Literature Cited
- Aedo J E, Fuentes-Valenzuela M, Molina A, and Valdés J A. . 2019. Quantitative proteomics analysis of membrane glucocorticoid receptor activation in rainbow trout skeletal muscle. Comp. Biochem. Physiol. Part D. Genomics Proteomics 32:100627. doi: 10.1016/j.cbd.2019.100627 [DOI] [PubMed] [Google Scholar]
- Borek C, Ong A, Mason H, Donahue L, and Biaglow J E. . 1986. Selenium and vitamin E inhibit radiogenic and chemically induced transformation in vitro via different mechanisms. Proc. Natl. Acad. Sci. U. S. A. 83:1490–1494. doi: 10.1073/pnas.83.5.1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottje W, Iqbal M, Tang Z X, Cawthon D, Okimoto R, Wing T, and Cooper M. . 2002. Association of mitochondrial function with feed efficiency within a single genetic line of male broilers. Poult. Sci. 81:546–555. doi: 10.1093/ps/81.4.546 [DOI] [PubMed] [Google Scholar]
- Bratic A, and Larsson N G. . 2013. The role of mitochondria in aging. J. Clin. Invest. 123:951–957. doi: 10.1172/JCI64125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks G A, Fahey T D, and Baldwin K M. . 2005. Exercise physiology: human bioenergetics and its applications. 4th ed. New York (NY): McGraw-Hill. [Google Scholar]
- Burdick N C, Banta J P, Neuendorff D A, White J C, Vann R C, Laurenz J C, Welsh T H Jr, and Randel R D. . 2009. Interrelationships among growth, endocrine, immune, and temperament variables in neonatal Brahman calves. J. Anim. Sci. 87:3202–3210. doi: 10.2527/jas.2009-1931 [DOI] [PubMed] [Google Scholar]
- Cafe L M, McIntyre B L, Robinson D L, Geesink G H, Barendse W, and Greenwood P L. . 2010. Production and processing studies on calpain-system gene markers for tenderness in Brahman cattle: 1. Growth, efficiency, temperament, and carcass characteristics. J. Anim. Sci. 88:3047–3058. doi: 10.2527/jas.2009-2678 [DOI] [PubMed] [Google Scholar]
- Cassar-Malek I, and Picard B. . 2016. Expression marker-based strategy to improve beef quality. Sci. World J. 2016:2185323. doi: 10.1155/2016/2185323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa P, Simões J A, Alves S P, Lemos J P, Alfaia C M, Lopes P A, Prates J A, Hocquette J F, Calkins C R, Vleck V, . et al. 2017. Beef palatability and its relationship with protein degradation and muscle fibre type profile in longissimus thoracis in Alentejana breed from divergent growth pathways. Animal 11:175–182. doi: 10.1017/S1751731116001373 [DOI] [PubMed] [Google Scholar]
- Cover S, Cartwright T C, and Butler O D. . 1957. The relationship of ration and inheritance to eating quality of the meat from yearling steers. J. Anim. Sci. 16:946–956. 10.2527/jas1957.164946x [DOI] [Google Scholar]
- Curley K O Jr., Paschal J C, Welsh T H Jr, and Randel R D. . 2006. Technical Note: Exit velocity as a measure of cattle temperament is repeatable and associated with serum concentration of cortisol in Brahman bulls. J. Anim. Sci. 84:3100–3103. doi: 10.2527/jas.2006-055 [DOI] [PubMed] [Google Scholar]
- Di Meo S, Napolitano G, and Venditti P. . 2019. Mediators of physical activity protection against ROS-linked skeletal muscle damage. Int. J. Mol. Sci. 20:3024. doi: 10.3390/ijms20123024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divakaruni A S, and Brand M D. . 2011. The regulation and physiology of mitochondrial proton leak. Physiology (Bethesda). 26:192–205. doi: 10.1152/physiol.00046.2010 [DOI] [PubMed] [Google Scholar]
- Fordyce G, Wythes J, Shorthose W, Underwood D, and Shepherd R.. 1988. Cattle temperaments in extensive beef herds in northern queensland. 2. Effect of temperament on carcass and meat quality. Aust. J. Exp. Agric. 28:689–693. doi: 10.1071/EA9880689 [DOI] [Google Scholar]
- Ghazi Harsini S, Habibiyan M, Moeini M M, and Abdolmohammadi A R. . 2012. Effects of dietary selenium, vitamin E, and their combination on growth, serum metabolites, and antioxidant defense system in skeletal muscle of broilers under heat stress. Biol. Trace Elem. Res. 148:322–330. doi: 10.1007/s12011-012-9374-0 [DOI] [PubMed] [Google Scholar]
- Gnaiger E, and MitoEAGLE Task Group 2020. Mitochondrial physiology. Bioenerg. Commun. 1:1–4. doi: 10.26124/bec:2020-0001.v1 [DOI] [Google Scholar]
- Haskell M J, Simm G, and Turner S P. . 2014. Genetic selection for temperament traits in dairy and beef cattle. Front. Genet. 5:368. doi: 10.3389/fgene.2014.00368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Jiao H, Song Z, Zhao J, Wang X, and Lin H. . 2015. Heat stress impairs mitochondria functions and induces oxidative injury in broiler chickens. J. Anim. Sci. 93:2144–2153. doi: 10.2527/jas.2014-8739 [DOI] [PubMed] [Google Scholar]
- Jastroch M, Divakaruni A S, Mookerjee S, Treberg J R, and Brand M D. . 2010. Mitochondrial proton and electron leaks. Essays Biochem. 47:53–67. doi: 10.1042/bse0470053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji L L. 2008. Modulation of skeletal muscle antioxidant defense by exercise: role of redox signaling. Free Radic. Biol. Med. 44:142–152. doi: 10.1016/j.freeradbiomed.2007.02.031 [DOI] [PubMed] [Google Scholar]
- Jurie C, Picard B, and Geay Y. . 1999. Changes in the metabolic and contractile characteristics of muscle in male cattle between 10 and 16 months of age. Histochem. J. 31:117–122. doi: 10.1023/a:1003589320910 [DOI] [PubMed] [Google Scholar]
- Kadim I T, Mahgoub O, Al-Marzooqi W, Khalaf S K, Mansour M H, Al-Sinani S S, and Al-Amri I S. . 2009. Effects of electrical stimulation on histochemical muscle fiber staining, quality, and composition of camel and cattle longissimus thoracis muscles. J. Food Sci. 74:S44–S52. doi: 10.1111/j.1750-3841.2008.00992.x [DOI] [PubMed] [Google Scholar]
- Kelly A K, Waters S M, McGee M, Fonseca R G, Carberry C, and Kenny D A. . 2011. mRNA expression of genes regulating oxidative phosphorylation in the muscle of beef cattle divergently ranked on residual feed intake. Physiol. Genomics 43:12–23. doi: 10.1152/physiolgenomics.00213.2009 [DOI] [PubMed] [Google Scholar]
- Kerth C R. 2013. Meat Tenderness. In: Kerth, C. R., editor. The science of meat quality. New York, NY: John Wiley & Sons, Inc. p. 99–117. [Google Scholar]
- Kim Y, Yang D S, Katti P, and Glancy B. . 2019. Protein composition of the muscle mitochondrial reticulum during postnatal development. J. Physiol. 597:2707–2727. doi: 10.1113/JP277579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King D A, Schuehle Pfeiffer C E, Randel R D, Welsh T H Jr, Oliphint R A, Baird B E, Curley K O Jr, Vann R C, Hale D S, and Savell J W. . 2006. Influence of animal temperament and stress responsiveness on the carcass quality and beef tenderness of feedlot cattle. Meat Sci. 74:546–556. doi: 10.1016/j.meatsci.2006.05.004 [DOI] [PubMed] [Google Scholar]
- Kühlbrandt W. 2015. Structure and function of mitochondrial membrane protein complexes. BMC Biol. 13:89. doi: 10.1186/s12915-015-0201-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S, Nielsen J, Hansen C N, Nielsen L B, Wibrand F, Stride N, Schroder H D, Boushel R, Helge J W, Dela F, . et al. 2012. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J. Physiol. 590:3349–3360. doi: 10.1113/jphysiol.2012.230185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham C M, Fenger C K, and White S H. . 2019. Rapid Communication: Differential skeletal muscle mitochondrial characteristics of weanling racing-bred horses. J. Anim. Sci. 97:3193–3198. doi: 10.1093/jas/skz203 [DOI] [PubMed] [Google Scholar]
- Li C, White S H, Warren L K, and Wohlgemuth S E. . 2016. Effects of aging on mitochondrial function in skeletal muscle of American American Quarter Horses. J. Appl. Physiol. (1985). 121:299–311. doi: 10.1152/japplphysiol.01077.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinild Lundby A-K, Jacobs R A, Gehrig S, de Leur J, Hauser M, Bonne T C, Flück D, Dandanell S, Kirk N, Kaech A, . et al. 2018. Exercise training increases skeletal muscle mitochondrial volume density by enlargement of existing mitochondria and not de novo biogenesis. Acta Physiologica. 222:e12905. doi: 10.1111/apha.12905 [DOI] [PubMed] [Google Scholar]
- Niki E, Noguchi N, Tsuchihashi H, and Gotoh N. . 1995. Interaction among vitamin C, vitamin E, and beta-carotene. Am. J. Clin. Nutr. 62(6 Suppl):1322S–1326S. doi: 10.1093/ajcn/62.6.1322S [DOI] [PubMed] [Google Scholar]
- Peternelj T T, and Coombes J S. . 2011. Antioxidant supplementation during exercise training: beneficial or detrimental? Sports Med. 41:1043–1069. doi: 10.2165/11594400-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Pfleger J, He M, and Abdellatif M. . 2015. Mitochondrial complex II is a source of the reserve respiratory capacity that is regulated by metabolic sensors and promotes cell survival. Cell Death Dis. 6:e1835. doi: 10.1038/cddis.2015.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, Taivassalo T, Gouspillou G, and Hepple R T. . 2011. Mitochondria: isolation, structure and function. J. Physiol. 589(Pt 18):4413–4421. doi: 10.1113/jphysiol.2011.212712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnampalam E N, Hopkins D L, Bruce H, Li D, Baldi G, and Bekhit A E-d. . 2017. Causes and contributing factors to “dark cutting” meat: current trends and future directions: a review. Compr. Rev. Food Sci. Food Saf. 16:400–430. doi: 10.1111/1541-4337.12258 [DOI] [PubMed] [Google Scholar]
- Ramos P M, Li C, Elzo M A, Wohlgemuth S E, and Scheffler T L. . 2020a. Mitochondrial oxygen consumption in early postmortem permeabilized skeletal muscle fibers is influenced by cattle breed. J. Anim. Sci. 98:1–10. doi: 10.1093/jas/skaa044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos P M, Wright S A, Delgado E F, van Santen E, Johnson D D, Scheffler J M, Elzo M A, Carr C C, and Scheffler T L. . 2020b. Resistance to pH decline and slower calpain-1 autolysis are associated with higher energy availability early postmortem in Bos taurus indicus cattle. Meat Sci. 159:107925. doi: 10.1016/j.meatsci.2019.107925 [DOI] [PubMed] [Google Scholar]
- Russell L A, Anderson D P, Paschal J C, and Young M. . 2016a. Price impacts of Brahman influence in southern Texas. Proceedings of the Southern Agricultural Economics Association 2016 Annual Meeting; February 6 to 9, 2016; San Antonio, TX. doi: 10.22004/ag.econ.229964 [DOI] [Google Scholar]
- Russell J R, Sexten W J, Kerley M S, and Hansen S L. . 2016b. Relationship between antioxidant capacity, oxidative stress, and feed efficiency in beef steers. J. Anim. Sci. 94:2942–2953. doi: 10.2527/jas.2016-0271 [DOI] [PubMed] [Google Scholar]
- Schiaffino S, and Reggiani C. . 2011. Fiber types in mammalian skeletal muscles. Physiol. Rev. 91:1447–1531. doi: 10.1152/physrev.00031.2010 [DOI] [PubMed] [Google Scholar]
- Spinazzi M, Casarin A, Pertegato V, Salviati L, and Angelini C. . 2012. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat. Protoc. 7:1235–1246. doi: 10.1038/nprot.2012.058 [DOI] [PubMed] [Google Scholar]
- St-Pierre J, Buckingham J A, Roebuck S J, and Brand M D. . 2002. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 277:44784–44790. doi: 10.1074/jbc.M207217200 [DOI] [PubMed] [Google Scholar]
- Talmant A, Monin G, Briand M, Dadet M, and Briand Y. . 1986. Activities of metabolic and contractile enzymes in 18 bovine muscles. Meat Sci. 18:23–40. doi: 10.1016/0309-1740(86)90064-1 [DOI] [PubMed] [Google Scholar]
- Thau L, and Sharma S. . 2020. Physiology, cortisol. Treasure Island (FL): Statpearls. [PubMed] [Google Scholar]
- Weber K, Brück P, Mikes Z, Küpper J H, Klingenspor M, and Wiesner R J. . 2002. Glucocorticoid hormone stimulates mitochondrial biogenesis specifically in skeletal muscle. Endocrinology 143:177–184. doi: 10.1210/endo.143.1.8600 [DOI] [PubMed] [Google Scholar]
- White S H, Warren L K, Li C, and Wohlgemuth S E. . 2017. Submaximal exercise training improves mitochondrial efficiency in the gluteus medius but not in the triceps brachii of young equine athletes. Sci. Rep. 7:14389. doi: 10.1038/s41598-017-14691-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S A, Ramos P, Johnson D D, Scheffler J M, Elzo M A, Mateescu R G, Bass A L, Carr C C, and Scheffler T L. . 2018. Brahman genetics influence muscle fiber properties, protein degradation, and tenderness in an Angus-Brahman multibreed herd. Meat Sci. 135:84–93. doi: 10.1016/j.meatsci.2017.09.006 [DOI] [PubMed] [Google Scholar]