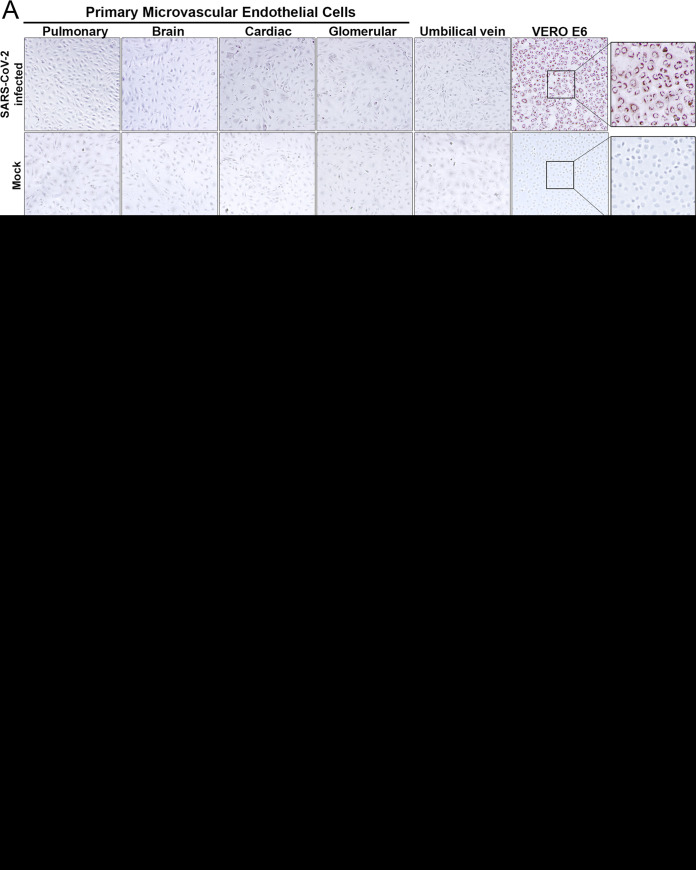
FIG 1.
SARS-CoV-2 fails to infect primary human endothelial cells without rACE2 expression. (A) Primary human microvascular endothelial cells from pulmonary (hPMECs), brain (hBMECs), cardiac (hCMECs), or glomerular (hGMECs) tissue or umbilical vein (HUVECs) or VeroE6 cells were mock or SARS-CoV-2 (strain WA) infected (multiplicity of infection [MOI] of 10) and at 24 hpi immunoperoxidase stained for nucleocapsid protein. (B and C) Primary human ECs and VeroE6, HEK293T, and Calu3 cells were analyzed by qRT-PCR for ACE2 mRNA (B) and by Western blotting for expressed ACE2 (C). HUVECs contain a potential ACE2 truncation lacking the N-terminal SARS-CoV-2 binding domain. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (D) Primary human pulmonary ECs lentivirus transduced to express recombinant ACE2 were infected with SARS-CoV-2 (MOI of 1) for 6 to 48 hpi. (E) ACE2-hPMECs or wild-type (WT)-hPMECs were immunoperoxidase stained for nucleocapsid protein. hPMECs or rACE2-hPMECs were analyzed by immunofluorescence assay (IFA) for the EC marker PECAM-1 and ACE2. (F) Following SARS-CoV-2 infection, rACE2-hPMECs were analyzed by IFA for coexpressed ACE2 and nucleocapsid protein (N) or PECAM-1 expression. Bars represent 50 μm. (G) For supernatants of SARS-CoV-2-infected (MOI of 1) WT hPMECs, hBMECs, rACE2-hPMECs, rACE2-hBMECs, and VeroE6 cells, titers were determined 2 to 72 hpi (limit of detection, <10 focus-forming units [FFU]/ml).