Abstract
Background
Hepatitis C virus (HCV) is considered as “Viral Time Bomb” suggested by the World Health Organization and if it is not treated timely, it will lead towards cirrhosis and hepatocellular carcinoma (HCC).
Objective
The purpose of the present research is to study possible risk factors, frequent genotypes of HCV and its association with different age groups.
Methods
Suspected blood samples from HCV patients were collected from different hospitals of Lahore, Pakistan. Out of 1000 HCV suspected samples, 920 samples were found HCV positive detected by Anti-HCV ELISA, CobasR. kit. The quantification of HCV load was determined by HCV quantification kit and LINEAR ARRAY KIT (Roche) was used for genotype determination by Real-Time PCR (ABI). Statistical analysis was done by using Microsoft Excel.
Results
Out of 920 subjects, 77 subjects (8.4%) were false positive and they were not detected by nested PCR. Three PCR positive samples were untypeable. Genotype 3 was predominant in Lahore which was 83.5%, whereas type 1 and 2 were 5.1% and 0.7% respectively. There were also mixed genotypes detected, 1 and 3 were 0.4%, 2 and 3 were 1.41% and 3 and 4 were 0.2% only. Male were more infected of HCV in the age <40 years and females >40years.
Conclusion
The major risk factor for HCV transmission is by use of unsterilized razors/blades. It is necessary to spread awareness among the general population of Pakistan about HCV transmission risk factors. Regular physical examination at least once a year is recommended, so that early detection of HCV could be done.
Keywords: Hepatitis C virus, hepatocellular carcinoma, quantification, genotype, real-time PCR
Introduction
Hepatitis C virus was discovered in 1989 which is enveloped by RNA and possesses 9.6 KB genome edged at both sides of the UTR region (5′ and 3′ ends). Hepatitis gene encodes 3000 amino acids of the polyprotein and post-translationally process take place that produces 3 structural proteins and 6 non- structural proteins 1. HCV has been recognized to be both hepatotropic and lymph tropic virus 2.
The chronic liver disease mainly caused by the HCV virus commonly progresses towards liver cirrhosis and hepatocellular carcinoma. HCV attributes 27% cirrhosis 3 and is the biggest reason for liver replacement 4.
HCV is categorized in 6 major genotypes on the basis of nucleotide variations. Genotype type 1 and genotype type 2 are primarily circulating around the world. In Pakistan, genotype 3 is primarily affected with 3a and 3b circulating with a similar pattern in males and females5–8. In Pakistan, already reported works to describe the occurrence of genotype 3a infections by use of unsterilized injections by inexperienced health workers mostly in backward areas 9–13. The occurrence of hepatitis C Virus in Pakistan is 57±17.7% among drug users 14. There is increased risk of HCV infection in drug addicts because of sharing injections and needles and unsafe sex practices 15–18.
Occurrence of HCV was also found, among non-injecting drug users, smokers, heroin, cocaine, crack, or methamphetamine users 19. HCV prevalence is 88% in street barbers 20. Virus from infected patients may be cleared out but they will be seropositive 21, 22. WHO guiding principle mentioned that polymerase chain reaction (PCR) is used to confirm HCV seropositivity and also a past infection 23. HCV is also transmitted through parental route, transfer or expose of the blood and their products, least effective transfer can be through sexual contact and from mother to baby. The 80% occurrence of HCV is linked with infection because of the custodial background when the first dose of the drug was injected. HCV spread by imprisonment 24, 25. The risk factors linked with HCV infection is, injecting for more than three years, injecting with person suffered with hepatitis and with sharing cotton 26. HCV is transferred by using syringes and single-use medicines more than one times on different patients 27, 28.
Materials and methods
Sampling and HCV positive samples detection
Thousand HCV suspected blood samples were collected from different hospitals of Lahore, Pakistan. Age of the patients was noted. Out of 1000 samples, 920 were found HCV positive by ELISA (Anti-HCV -Antibody to hepatitis C virus, Anti-HCV, Cobas R kit) by E-170 Roche instrument. Total RNA from the patients sera were extracted by GF Vivantis (nucleic acid extraction kit). Reverse Transcriptase PCR technology was used for the qualitative determination of HCV ELISA positive samples. Nested PCR was performed and all the procedure and recipe was provided with the master mixture (Fermentas, technologies USA), the PCR products were run on 2% agarose gel and the bands were visualized under gel doc (BioRad).
HCV quantification
HCV positive RNA samples were quantified by real-time PCR machine, International Applied Biosystems (ABI) by using HCV quantification kit (Geno Sacace, Biotechnologies, Italy), by using primer probes chemistry. The lower to upper detection limit was 250 IU/mL to 5.0×108 IU/mL respectively. The samples having the above limit than upper were diluted and then the quantification values obtained by real-time PCR were multiplied by their respective dilution factor and the actual amount of HCV RNA was determined in IU/mL.
HCV genotyping by real-time PCR
The HCV positive, PCR samples were subjected for genotype determination and Geno-Sen's HCV Genotyping 1/2/3/4 real-time PCR Kit for Rotor Gene 2000/3000/6000, Genome diagnostics Private LTD was used. The kit had four types of tubes of different colors, blue, yellow, red and white. Blue cap tube having R1 solution (HCV genotyping supermix), Yellow cap dye R2 tube had Mg++ sol for real-time PCR, Red cap tube had HCV genotype positive control only for HCV 1/2/3/4 Genotypes. 7.5uL R1 solution and 2.5uL R2 solution was mixed, later 15uL RNA (50ng/uL) or standard was mixed for 25uI reaction mixture. The PCR cycling conditions were 95°C for 15 seconds, 55°C for 20 seconds and 72°C for 15 seconds.
Statistical analysis
Statistical analysis was done by using Microsoft Excel. To determine the differences between two variables t-test was performed, the confidence interval was 95% with 0.5 % error. Therefore, the p-value less than 0.05 was considered as significant and the p-value more than 0.05 was considered as non-significant.
Results
Out of 1000 suspected HCV samples, 920 samples were found ELISA positive and while 843 samples were found positive by nested PCR and quantified by real-time PCR. So the 77 samples were false positive by ELISA and 3 samples were untypable by HCV Genotyping 1/2/3/4 real-time PCR Kit for Rotor Gene 2000/3000/6000. Different dyes were used for different genotypes. Cy5 Channel for Genotype 1, Fam Channel for Genotype 2, Rox Channel for genotype 3, Joe Channel Genotype 4, when there was not any signal among these channels then it was named as untypable genotype.
Age-wise distribution of genotype
Nineteen samples of less than 20 years were HCV ELISA positive. Out of which 14 males and 5 female's samples were positive. While one sample from each group was PCR negative and seventeen samples were typable. Only genotype 1 and 3 were reported in males 15.5% and 84.6% respectively, while only genotype 3 (100%) was pre-dominant in females of this age group figure 1. In this age group, males were more affected with HCV and HCV false-positive ELISA results were more in females.
Figure 1.
HCV Detection and genotype distribution in the age group of less than 20 years.
Almost 34.13 % (314) samples of age group 21–40 years were HCV ELISA positive. Out of which 197 males and 117 females samples were positive, while 12 males and 14 females were PCR negative and 288 samples were typable. Genotype 1 (5%), 2 (1%), 3 (93%), 2&3 (1%) were present in males while 1 (3%), 2 (2%), 3 (92%), 1&3(1%) and 2&3(1%), were present in females. In this age group again males were more affected with HCV and HCV false-positive ELISA results were more in females (Figure 2).
Figure 2.
HCV Detection and Genotype distribution in age group 21–40 years
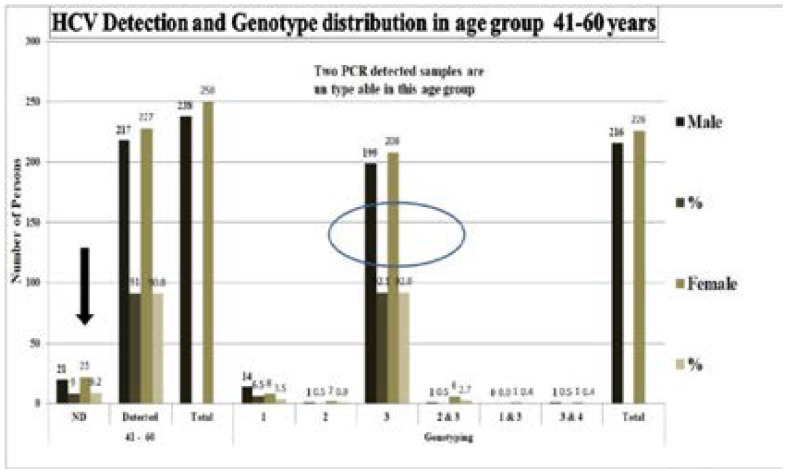
Almost 53.04 % (488) samples of age group 41–60 years were HCV ELISA positive. Out of which 238 males and 250 females were positive, while 20 males and 22 females were PCR negative and 446 samples were typable. Genotype 1 (6.5%), 2 (0.5%), 3 (92.1%), 2&3(0.5%),1&3(0%), 3&4 (0.5%) were present in males while 1 (3.5%), 2 (0.9%), 3 (92%), 1&3(0.4%) and 2&3(2.7%), 3&4(0.4%) were present in females. In this age group males were less affected with HCV which is different from previous two groups and HCV false positive ELISA results were more in females (Figure 3).
Figure 3.
HCV Detection and genotype distribution in age group 41–60 years
In this age group 100 out of 920 (10.87%) HCV ELISA positive samples, Out of which 1 male and 3 females were PCR negative. While 58 females and 38 males were PCR positive were typable. Genotypes, 1 (2.7%), 2 (0.0%),3 (91.9%), 2&3(5.4%), 1&3(0%), 3&4 (0%) were present in males while genotypes, 1 (15.5%),2 (0.0%), 3 (79.3%), 2&3(1.7%) and 1&3(3.4%), 3&4(0.0%) were present in females. In this age group males were less affected with HCV which is different from age groups<40 and HCV false positive ELISA results were more in females (Figure 4).
Figure 4.
HCV Detection and Genotype distribution in age group more than 60 years
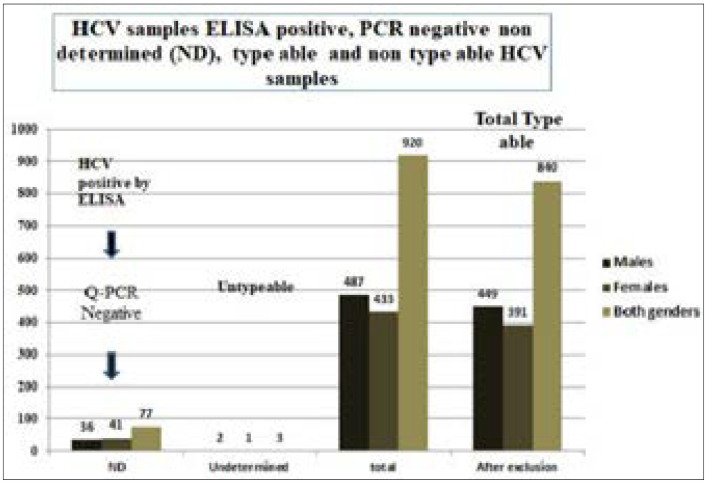
Figure 5 shows that 77 samples were PCR negative which was ELISA positive and 3 samples were undetermined by genotyping 1/2/3/4 CorbasR Kit. 391 females and 449 males were typable, which made 91.3% of total ELISA positive samples, while 91.63% was PCR positive (Figure 5).
Figure 5.
HCV samples ELISA positive, PCR negative, non determined (ND), type able and non type able HCV samples
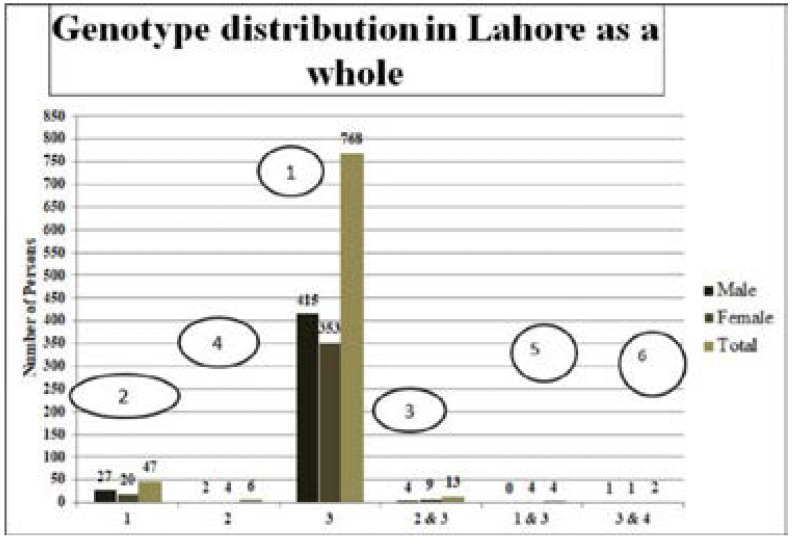
The most prevalent genotype in Lahore in both genders were 3 (768, 91.42%), 1(47, 5.59%), 2&3 (13, 1.55%), 2(6, 0.71%), 1&3 (4, 0.48%), 3&4 (2, 0.24%), respectively (Figure 6).
Figure 6.
Genotype distribution in Lahore as a whole
Total 843 samples PCR positive samples were tested for genotyping, three samples were untypable and were in the age groups >40 years, 2 samples from males and one from the female.
The remaining 840 type able samples of both genders showed different number of HCV positive samples, 442(52.61%) HCV PCR positive type able samples of age group 41–60 years were at the top, leading towards 21–40 years age group with 286 (34.05%), >60 years age group, 95 (11.30%) and the least <20 years age group with 17(2.02%) PCR positive typable patients, respectively. There was non-significant difference of the number of samples in males and females with p-value 0.568327, although apparently, males as a whole showed more numbers of HCV positive patients (Figure 7) as compared to females.
Figure 7.
Age-wise distribution of HCV type able samples
The HCV titer below 60,000 IU/mL was considered as low titer while between 60,000–80,000 IU/mL was considered as intermediate. HCV titer above 80,000 IU/mL was considered as high titer. All the typable PCR samples had titer between 20,000–70,000IU/mL and the untypale HCV sample had a titer of 70,000–80,000IU/mL, which showed that these samples had the higher titer than typable HCV samples. So, the untypability was due to changes in genotypes which were not detectable by the real-time PCR genotyping kit 1/2/3/4 Corbas R. Untypability was not due to low viral titer but due to variations of sequences in already reported genotypes. The patients were asked for the probable risk factor of HCV infection and the table of risk factors was prepared (Table 1) and the possible % age distribution of HCV risk factor is also shown in figure 8. The most common genotype in the group is 3 and the most common route of transmission are needles/syringes, the 2nd most is the medical/dental surgeries while genotype 1 is the second common genotype in Lahore although it is 6.12% of 3 genotypes circulating in Lahore and the major risk factor for this is the barbers shop, medical/dental surgeries, blood transfusion and the remaining g is unknown. The genotype distribution is also described in fig 6. and as a whole the 50% of HCV PCR positive samples had a risk factor of misuse of needles and syringes, 20% were due to Medical/ dental Surgeries, 13% due to lack of caring of barbers, 10% due to blood transfusions and 7% due to unknown reasons (Figure 8).
Table 1.
HCV risk factors %age distribution
Figure 8.
HCV Risk factor % age distribution
Discussion
According to the World Health Organization HCV is considered a viral time bomb, which is killing the life of many people and creating difficulties in treatment because of different genotypes of HCV. Different reports have shown that different countries have a prevalence of different genotypes due to which different treatment pattern should be adopted. HCV risk factors are differently reported for different HCV genotypes. So, it is very necessary to study the prevalence of HCV genotypes in different populations of the world and even among the different ethnicity of the same country.
In this research work, 920 ELISA positive samples were collected from different diagnostics laboratories and hospitals of Lahore. The complete history of patients was taken. HCV qualitative test was performed by real-time PCR and it was found that out of 920 ELISA positive samples 843 were PCR positive samples, it means 77 (8%) patients had false-positive ELISA results. Different studies have shown that ELISA test gives false-positive results 29, 30, so this test is not so much reliable and it is necessary to perform nested PCR or real-time PCR to find out the exact copy numbers of HCV. HCV quantification was performed by HCV quantification kit (Geno Sacace, Biotechnologies, Italy) and LINEAR ARRAY KIT (Roche) was used for HCV genotyping by real-time PCR. Out of 843 samples, 800 samples gave typing results while 3 samples were untyped. This may be due to other genotypes 5, 6 or maybe due to unknown HCV genotypes. So, it is also necessary to design a kit having all possible genotypes in the kit, so that we can discriminate the less occurring known and unknown genotypes by real-time kit method. HCV PCR positive samples were more in males in the age group <40 years as compared to HCV positive samples of females while vice versa for females. HCV positive samples were more frequent in 41–60 years of the age group in both genders, but as a whole, there was not any statistical difference in HCV positivity in different age groups and sex (p>0.5), the same results have been obtained by other different studies 31–33. HCV genotype 3 was more prevalent in patients then 1, 2& 3, 2, 1&3 and 3& 4 respectively.
The most prevalent genotype 3 is circulating in Pakistan and the same results were reported by different ethnic groups of Pakistan 31, 34–45. Patients having genotype 3 have more chances of cure 46, 47 as it has a shorter time period for its cure as compared to genotype 1 and 2, but it is alarming that other genotypes are also increasing in Pakistan even the untyped ones which have the longer time period of cure 49. The predominance of HCV in Pakistan also shows that its nearest countries have the same genotypes, which is also reported by other researchers, like Iran, Bangladesh, China and India respectively 44, 49–55. Genotype 1 is common in China and Iran 56 and also in Europe and Japan 57. In our study, we also have found untypable genotypes and the same type of results have been obtained from other studies of Pakistan 41, 42, 44. The [sa10]mode of transmission for genotype 3 is by contaminated needles and syringes and the second most prevalent transmission route was dental/medical surgeries and the same results were obtained by other studies in Pakistan45.
The medical doctors and dentists are using unsterilized instruments and spreading the disease among healthy persons 37. The third mode of transmission identified in our studies was from barbers shops because they are reusing the same razors on different customers58 and the 4th one was by blood transfusions. The blood transfusions can cause various types of diseases and this is also considered as one of the major risk factors of HCV infection in Pakistan as well 41 and the remaining patients did not describe any reasons of HCV infection. So, it is also necessary to find out the other modes of infections of HCV.
Conclusion
HCV infection is spreading day by day. HCV detection by ELISA may give false-positive results, more accurate ELISA based methods should be developed. Conventional PCR and real-time PCR based detection methods are more reliable. The genotype 3a is the most common type of HCV genotype in Lahore and other genotypes 1 and 2 are also increasing day by day, having more time period of treatment or no response to treatment many times, so it is necessary to avoid the spread of these later mentioned deadly genotypes in Pakistan. Untypable genotypes are also found in Lahore. The real-time genotyping kit is very specific and reliable, it is necessary to include the known genotypes 5, 6 in the kit and the unknown genotypes as well after sequencing of unknown genotypes. The main mode of transmission is the use of unsterilized needles and syringes. The HCV infection rate is almost the same in males and females and proper prevention measures should be adopted by spreading the awareness among the general population of Pakistan about HCV transmission risk factors. Regular physical examination at least after every year is recommended, so that early detection of HCV could be done and proper treatment measures would be adopted which would definitely decrease the death rate due to HCV infection in Pakistan.
Conflicts of interest
There is no conflict of interest among the authors.
References
- 1.Argentini C, Genovese D, Dettori S, Rapicetta M. HCV genetic variability from quasi species evolution to genotype classification. Future Microbiol. 2009;4:359–373. doi: 10.2217/fmb.09.8. [DOI] [PubMed] [Google Scholar]
- 2.Akhund AA, Shaikh KR, Naqvi SQH, Kamal M. HCV Genotype in correlation to histopathtological grading and staging in interior Sindh. Gomal J of Medical Sciences. 2008;6(2):93–97. [Google Scholar]
- 3.Zaman N, Asad MJ, Raza A, Raja GK, Akhter S, Mahmood M, Mahmood RT. Detection of HCV RNA and NS5A protein in peripheral blood mononuclear cells after sustained virological response may cause viral relapse. Pakistan Journal of Zoology. 2015;47(4) [Google Scholar]
- 4.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. Journal of hepatology. 2006;45(4):529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Waheed Y. Effect of interferon plus ribavirin therapy on hepatitis C virus genotype 3 patients from Pakistan: treatment response, side effects and future prospective. Asian Pacific J of Tropical Med. 2015;8(2):859. doi: 10.1016/S1995-7645(14)60193-0. [DOI] [PubMed] [Google Scholar]
- 6.Ali A, Nisar M, Ahmad H, Saif N, Idrees M, Bajwa M. Determination of HCV genotypes and viral loads in chronic HCV infected patients of Hazara Pakistan. Virol J. 2011;8:466. doi: 10.1186/1743-422X-8-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan T, Mehr M, Ullah H, Khan H, Iman N. Frequency of hepatitis C genotypes in the north of Pakistan. Gomal J Med Sci. 2014;12:9–106. [Google Scholar]
- 8.Akhtar M, Majeed S, Jamil M, Rehman A. Hepatitis C virus infection among injecting drug users in Lahore, Pakistan: a cross sectional study. Pak J Med Sci. 2016;32(2):373–378. doi: 10.12669/pjms.322.9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Safi A, Waheed Y, Sadat J, ul lslam S, Salahuddin S, Saeed U, Ashraf M. Molecular study of HCV detection, genotypes and their routes of transmission in north west Frontier Province, Pakistan. Asian Pac J Trop Biomed. 2012;2:532–536. doi: 10.1016/S2221-1691(12)60091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Umar M, Ahmad M, Khurram M, Usman S, Arif M, Adam T, Minhas Z, Arif A, Naeem A, Ejaz K, et al. Hepatitis C in Pakistan: a review of available data. Hepat Mon. 2010;10(3):205–214. [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmad N, Asgher M, Shafique M, Qureshi J. An evidence of high prevalence of hepatitis C virus in Faisalabad, Pakistan. Saudi Med J. 2007;8(3):5–390. [PubMed] [Google Scholar]
- 12.Alam S, Ahmad N, Khan M, Mustafa G, Al Mamun A, Mashud G. Sero prevalence of hepatitis C virus infection among health care workers. J Bangladesh College of Physicians and Surgeons. 2007;25(3):126–129. [Google Scholar]
- 13.Khan N, Akmal M, Hayat M, Umar M, Ullah A, Ahmed I, Rahim K, Ali S, Bahadar S, Saleha S. Geographic distribution of hepatitis C virus genotypes in Pakistan. Hepat Mon. 2014;14(10):1–5. doi: 10.5812/hepatmon.20299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waheed Y, Shafi T, Safi SZ, Qadri I. Hepatitis C virus in Pakistan: a systematic review of prevalence, genotypes and risk factors. World J Gastroenterol. 2009;15:5647–5653. doi: 10.3748/wjg.15.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howe CJ, Fuller CM, Ompad DC, Galea S, Klobin B, Thomas D, et al. Association of sex, hygiene and drug equipment sharing with hepatitis C virus infection among non-injecting drug users in New York City. Drug Alcohol Depend. 2005;79(3):389–395. doi: 10.1016/j.drugalcdep.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Jittiwutikarn J, Thongsawat S, Suriyanon V, Maneekarn N, Celentano D, Razak MH, et al. Hepatitis C infection among drug users in northern Thailand. Am J Trop Med Hyg. 2006;74(6):1111–1116. [PubMed] [Google Scholar]
- 17.Macías J, Palacios RB, Claro E, Vargas J, Vergara S, Mira JA, et al. High prevalence of hepatitis C virus infection among noninjecting drug users: association with sharing the inhalation implements of crack. Liver Int. 2008;26:781–786. doi: 10.1111/j.1478-3231.2008.01688.x. [DOI] [PubMed] [Google Scholar]
- 18.Segurado AC, Braga P, Etzel A, Cardoso MRA. Hepatitis C virus coinfection in a cohort of HIV-infected individuals from Santos, Brazil: sero prevalence and associated factors. AIDS Patient Care STDS. 2004;18(3):135–143. doi: 10.1089/108729104322994829. [DOI] [PubMed] [Google Scholar]
- 19.Stern RK, Hagan H, Lelutiu-Weinberger C, Jarlais DD, Scheinmann R, Strauss S, et al. The HCV Synthesis Project: Scope, methodology, and preliminary results. BMC Med Res Methodol. 2008;8:62. doi: 10.1186/1471-2288-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuo I, Salman ul-Hasan Galai N, Thomas DL, Zafar T, Ahmed MA, et al. High HCV seroprevalence and HIV drug use risk behaviors among injection drug users in Pakistan. Harm Reduct J. 2006;3:26. doi: 10.1186/1477-7517-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerlach JT, Diepolder HM, Zachoval R, Gruener NH, Jung MC, Ulsenheimer A, Schraut WW, Schirren CA, Waechtler M, Backmund M, Pape GR. Acute hepatitis C: high rate of both spontaneous and treatment-induced viral clearance. Gastroenterology. 2003;125:808. doi: 10.1016/s0016-5085(03)00668-1. [DOI] [PubMed] [Google Scholar]
- 22.Thomson EC, Fleming VM, Main J, Klenerman P, Weber J, Eliahoo J, Smith J, McClure MO, Karayiannis P. Predicting spontaneous clearance of acute hepatitis C virus in a large cohort of HIV-1-infected men. Gut. 2011;60:837–845. doi: 10.1136/gut.2010.217166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO Guidelines Approved by the Guidelines Review Committee, author. Guidelines for the Screening, Care and Treatment of Persons with Hepatitis C Infection. Geneva: World Health Organization; 2014. [PubMed] [Google Scholar]
- 24.Kheirandish P, Alinaghi SAS, Jahani MR, Shirzad H, Ahmadian MRS, McFarland Willi, et al. Prevalence and Correlates of Hepatitis C Infection among Male Injection Drug Users in Detention, Tehran, Iran. J Urban Health. 2009;86(6):902–908. doi: 10.1007/s11524-009-9393-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SS. Prevalence of hepatitis C infection in injection drug users in Hong Kong. Hong Kong Med J. 2009;15(Suppl 8):S45–S46. [PubMed] [Google Scholar]
- 26.Diaz T, Vlahov DCD, Perlis TE, Edwards V, Friedman SR, Rockwell R, Hoover D, Williams IT, Monterrosoet ER. Factors Associated With Prevalent Hepatitis C: Differences Among Young Adult Injection Drug Users in Lower and Upper Manhattan, New York City. Am J Pub Health. 2001;91(1):20–23. doi: 10.2105/ajph.91.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.CDC 2007. (Centers for Disease Control and Prevention), author Acute hepatitis C virus infections attributed to unsafe injection practices at an endoscopy clinic--Nevada, 2007 in Hepatology. 2008;48(4):1333–1335. [PubMed] [Google Scholar]
- 28.Alavian SM. We need a new national approach to control hepatitis c: It is becoming too late. Hepatitis Monthly. 2008;8(3):165–169. [Google Scholar]
- 29.Moorman AC, Drobenuic J, Kamili S. Prevalence of false-positive hepatitis C antibody results, National Health and Nutrition Examination Study (NHANES) 2007–2012. J Clin Virol. 2017;89:1–4. doi: 10.1016/j.jcv.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ioniţă E, Lupulescu E, Alexandrescu V, Chiriţă C, Bălteanu M, Neguţ AE. False-positive ELISA reactions for the hepatitis C virus and the human immunodeficiency virus after anti-influenzal vaccination. Bacteriol Virusol Parazitol Epidemiol. 1995;40(3–4):249–252. [PubMed] [Google Scholar]
- 31.Inamullah, Idrees M, Ahmed H, Ali M, Ali L, Ahmed A. Hepatitis C virus genotypes circulating in district Swat of Khyber Pakhtoonkhaw, Pakistan. Virol J. 2011;8:16. doi: 10.1186/1743-422X-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kabir A, Alavian S, Keyvani H. Distribution of hepatitis C virus genotypes in patients infected by different sources and its correlation with clinical and virological parameters: a preliminary study. Comp Hepatol. 2006;5:4. doi: 10.1186/1476-5926-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raja NS, Janjua KA. Epidemiology of Hepatitis C virus infection in Pakistan. Immunol J M Infect. 2008;41:4–8. [PubMed] [Google Scholar]
- 34.Attaullah Sobia, Khan Sanaullah, Ali Ijaz. Hepatitis C virus genotypes in Pakistan: a systemic review. Virol J. 2011;8:433. doi: 10.1186/1743-422X-8-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Afridi SQ, Zahid MN, Shabbir MZ, Hussain Z, Mukhtar N, Tipu MY, et al. Prevalence of HCV genotypes in district Mardan. Virol J. 2013;10:90. doi: 10.1186/1743-422X-10-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Idrees M, Riazuddin S. Frequency distribution of hepatitis C virus genotypes in different geographical regions of Pakistan and their possible routes of transmission. BMC Infect Dis. 2008;8:69. doi: 10.1186/1471-2334-8-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Attaullah S, Khan S, Ali I. Hepatitis C virus genotypes in Pakistan: a systemic review. Virol J. 2011;8:433. doi: 10.1186/1743-422X-8-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ali A, Nisar M, Ahmad H, Saif N, Idrees M, Bajwa M. Determination of HCV genotypes and viral loads in chronic HCV infected patients of Hazara Pakistan. Virol J. 2011;8:466. doi: 10.1186/1743-422X-8-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan T, Mehr M, Ullah H, Khan H, Iman N. Frequency of hepatitis C genotypes in the north of Pakistan. Gomal J Med Sci. 2014;12:9–106. [Google Scholar]
- 40.Safi A, Waheed Y, Sadat J, ul lslam S, Salahuddin S, Saeed U, Ashraf M. Molecular study of HCV detection, genotypes and their routes of transmission in north west Frontier Province, Pakistan. Asian Pac J Trop Biomed. 2012;2:532–536. doi: 10.1016/S2221-1691(12)60091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ali S, Ali I, Azam S, Ahmad B. Frequency distribution of HCV genotypes among chronic hepatitis C patients of khyber pakhtunkhwa. Virol Journal. 2011;8 doi: 10.1186/1743-422X-8-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saleha S, Kamal A, Ullah U, Khan N, Mahmood A, Khan S. Prevalence of of hepatitis C virus genotypes in district Bannu, Khyber Pakhtunkhwa, Pakistan. Hepat Res and Treatment. 2014;2014:1–5. doi: 10.1155/2014/165826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khan S, Attaullah S, Ayaz S, Khan N, Shams S, Ali I, Bilal M, Siraj S. Molecular epidemiology of HCV among health care workers of Khyber Pakhtunkhwa. Virol Journal. 2011;8:105. doi: 10.1186/1743-422X-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahmad W, Ijaz B, Javed F, Jahan S, Shahid I, Khan F, Hassan S. HCV genotype distribution and possible transmission risks in Lahore, Pakistan. World J Gastroenterol. 2010;16:4321–4328. doi: 10.3748/wjg.v16.i34.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saeed U, Manzoor S. Risk factors associated with transmission of hepatitis B and hepatitis C virus in Pakistan. Glob J of Medi resear Dise. 2014;14:2249–4618. [Google Scholar]
- 46.Ahmad S, Salati SAA, Mattar EH, Al-Sabban AMH, Hamad AM. Epidemiology of Hepatitis C Virus (HCV) Infection. Physicians Academy. 2010;4(8):82–87. [Google Scholar]
- 47.Qazi MA, Fayyaz M, Chaudhary GMD, Jamil A, Malik AH, Gardezi AI, Bukhari MH. Hepatitis C virus genotypes in Bahawalpur. Biomedica. 2006;22:51–54. [Google Scholar]
- 48.Abbas SZ, Al M, MuhammaD AH, Shaw S, Abba SQ. Frequency of HCV infection and its genotypes among patients attending a liver clinic and voluntary blood donors in a rural area of Pakistan. Pak J Med Sci. 2009;25(4):579–582. [Google Scholar]
- 49.Husain A, Malik FA, Nagra H, Ehsan A, Ahmad Z, Abid M. Frequency of Different HCV Genotypes in Faisalabad. A P M C. 2009;3(1):19–22. [Google Scholar]
- 50.Qazi MA, Fayyaz M, Chaudhary GMD, Jamil A, Malik AH, Gardezi AI, Bukhari MH. Hepatitis C virus genotypes in Bahawalpur. Biomedica. 2006;22:51–54. [Google Scholar]
- 51.Ijaz T, Shahzad MK, Sarfraz N, Khan MA. Prevalence of Genotype 3a Hepatitis C Virus (HCV) In the Infected Population of Lahore, Pakistan. IJAVMS. 2008;2:145–147. [Google Scholar]
- 52.Ziyaeyan M, Alborzi A, Jamalidoust M, Badiee P, Moeini M, Kadivar A. Prevalence of hepatitis C virus genotypes in chronic infected patients, southern Iran. JJM. 2011;4(3):141–146. [Google Scholar]
- 53.Sanders-Buell E, Rutvisuttinunt W, Todd CS, Nasir A, Bradfield A, Lei E, et al. Hepatitis C genotype distribution and homology among geographically disparate injecting drug users in Afghanistan. J Med Virol. 2013;85(7):1170–1179. doi: 10.1002/jmv.23575. [DOI] [PubMed] [Google Scholar]
- 54.Das BR, Kundu B, Khandapkar R, Sahni S. Geographical distribution of hepatitis C virus genotypes in India. Indian J Pathol Microbiol. 2002;45(3):323–328. [PubMed] [Google Scholar]
- 55.Cui Y, Jia J. Update on epidemiology of hepatitis B and C in China. J Gastroenterol Hepatol. 2013;28(Suppl 1):7–10. doi: 10.1111/jgh.12220. [DOI] [PubMed] [Google Scholar]
- 56.Jahanbakhsh Sefidi F, Keyvani H, Monavari SH, Alavian SM, Fakhim S, Bokharaei-Salim F. Distribution of hepatitis C virus genotypes in Iranian chronic infected patients. Hepat Mon. 2013;13(1) doi: 10.5812/hepatmon.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rouabhia S. Hepatitis C virus genotypes in north eastern Algeria: a retrospective study. World J J Hepatol. 2013;5:393–397. doi: 10.4254/wjh.v5.i7.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bari A, Akhtar S, Rahbar MH, Luby SP. Risk factors for hepatitis C virus infection in male adults in Rawalpindi-Islamabad, Pakistan. Trop Med Int Health. 2001;6:732–738. doi: 10.1046/j.1365-3156.2001.00779.x. [DOI] [PubMed] [Google Scholar]