ABSTRACT
Matrine is one of the major alkaloids extracted from Sophora flavescens Ait of the traditional Chinese medicine, was the main chemical ingredient of compounds of Kushen injection. The Matrine is considered as a promising therapeutic agent for curing nonsmall cell lung cancer (NSCLC), used either alone or combined with chemotherapeutic agents. In the present study, we focused on the possible roles of Matrine exerted on the self-renewal ability of stem-like cells of the NSCLC group, as well as the cytotoxicity of chemotherapeutic agents, in vitro and in vivo. Here we reported that Matrine inhibits cancer stem-like cell (CSC) properties through upregulation of Let-7b and suppression of the Wnt pathway. Overexpression of Let-7b suppressed the ability of tumorsphere formation, decreased Wnt pathway activation through inhibiting its transcriptional activity in lung CSCs. Further studies revealed that Let-7b directly targeted CCND1 and decreased its expression, whereas Matrine increased Let-7b levels and followed by inactivation of the CCND1/Wnt signaling pathway and inhibition of EMT, which was characterized by loss of epithelial markers and acquisition of a mesenchymal phenotype in lung CSCs. What is more, we found that Matrine increased Let-7b level in an endoribonuclease DICER1-dependent manner. And xenografts in nude mice evidenced that Matrine increased the sensitivity of lung CSCs to 5-FU and inhibited the accumulation of CCND1 in tumor tissues induced by 5-FU. Taken together, these data illustrate the role of Let-7b in regulating lung CSCs traits and DICER1/let-7/CCND1 axis in Matrine or in combination with 5-FU intervention of lung CSCs’ expansion, helping to fulfill the anti-cancer action of Matrine.
KEYWORDS: Matrine, therapy resistance, NSCLC, cancer stem cells, division manners
Introduction
Matrine is the major bioactive component among the kinds of Sophora flavescens Ait, which was the main chemical ingredient of compounds of Kushen injection. Its chemical formula is C15H24N2O [1]. Matrine has been extensively used either alone or combined with chemotherapeutic agents or radiotherapy in Chinese clinical settings for many years [2]. Matrine has a good medicinal effect as an alkaloid. Its antiviral activity has broad application prospects in the treatment of chronic hepatitis B [3]. It has been reported that Matrine and its compounds inhibits the growth and proliferation of various cancer cells through inducing apoptosis and cell cycle arrest [4–6]. Matrine can also inhibit tumor cell migration, invasion, and adhesion by downregulating the expression of some active oncogenes [7–9]. However, the antitumor effect of Matrine on NSCLC and its molecular mechanism have not been fully elucidated.
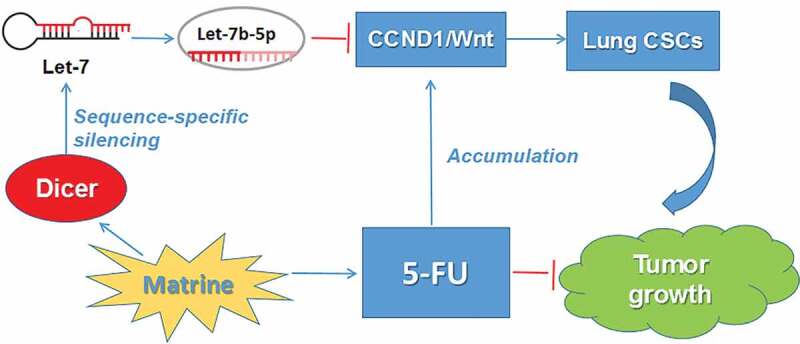
Lung cancer is the most common cause of cancer and cancer death in China, accounting for 21% of all cancers and 27% of all cancer-related deaths, of which NSCLC represents approximately 80% [10].Although chemotherapy can prolong survival in patients with advanced diseases, clinically significant side effects can reduce its efficacy, as there are often too many reports of toxicity [11]. As an important posttranscriptional regulator of gene expression, microRNAs play an important role in tumorigenesis. The let-7 family of microRNAs, traditionally considered as tumor suppressors, targets, and degrades downstream oncogenes by binding to Argonaute to form RNA-induced silencing complexes (RISC) [12,13]. Wnt signaling pathway is an evolutionarily conserved signaling pathway that regulates cell development by controlling cell fate, cell proliferation, differentiation, and cell apoptosis [14–16]. As a downstream target of Wnt, cyclin-D 1 (encoded by CCND1), a cell-cycle regulatory protein is another indicator of poor prognosis in NSCLC [17]. Epithelial mesenchymal transition (EMT) is a phenotypic transformation of epithelial cells into mesenchymal cells. In the course of tumor progression, tumor cells become more aggressive after EMT and get access to the blood vessels leading to distant metastasis, which is the main cause of cancer death. It has been proved that the Wnt signaling pathway is crucial to EMT, blocking Wnt signaling attenuates EMT [18]. The aim of this study was to investigate the therapeutic effect and molecular mechanism of Matrine on NSCLC CSCs. We found that Let-7b expression in the Let-7 family increased most significantly after Matrine treatment and the key role of Let-7b in regulating lung CSC traits. Then we elucidated that Matrine effectively inhibited the expression of CCND1/Wnt through up-regulating of Let-7b. In addition, by combining with other chemotherapeutic agents, Matrine effectively abolishes CSCs properties. Our results support further evaluation of Matrine or derivatives as therapeutic agents for NSCLC.
Materials and methods
Cell culture, transient transfection, and reagents
Two human NSCLC cell lines, NCI-A549 and NCI-H460 were obtained from Shanghai Cell Bank of Chinese Academy of Sciences. A549 were maintained in DMEM medium (Gibco, Carlsbad, CA, USA), while H460 were cultured in RPMI 1640 medium (Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin). EGF, bFGF and B27 were purchased from Invitrogen (Carlsbad, CA). Matrine (Lot NoMKBS8661V) were purchased from Sigma.
Antibodies against KLF4, CD133 were acquired from Abcam (Cambridge, UK). E-cadherin, N-cadherin, and Vimentin were from Cell Signaling Technology, Inc (Massachusetts, US). CCND1 and TCF4 were from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). MiRNAs and vectors were transfected using Lipofectamine 2000 reagent (Invitrogen). The Let-7b mimic, negative control were purchased from GenePharma (Suzhou, China).
MTT assay
The Log-phase growing cells were seeded into 96-well plates at a density of 6 × 103 cells/well in media. The cells were exposed to Matrine at concentrations, 0 (negative control), 0.6, 1.0, 1.4 mg/ml for 24 h, 48 h, and 72 h at 37°C. Cells viability was measured using 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assays.
Colony formation assay
1 × 103 cells were seeded in six-well plates at a single-cell density, and the cells were treated with the indicated drugs, subsequently grown for 2 weeks. Colonies containing more than 50 cells were counted after staining with crystal violet (Solarbio).
Tumorsphere formation assay
Cells were seeded into ultra-low-attachment six-well plates at a density of 1 × 104 cells/well in serum-free medium (SFM) (DMEM/F-12) (Gibco) supplemented with 20 ng/mL EGF, 20 ng/mL bFGF, and B27. Different concentrations of Matrine (0.6, 1.0, and 1.4 mg/mL) were added to SFM. After 10 days of culture, the size and number of the tumorspheres were obtained under a Nikon Eclipse TE2000-S microscope.
Western blot analysis
Cells were lysed in RIPA buffer supplemented with protease inhibitors. Equal amounts of protein were subject to SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA). After blocking with 5% nonfat milk, membranes were incubated with the indicated antibodies and detected by enhanced chemiluminescence reagents.
Immunofluorescence staining
Cells were grown on glass slides and treated with Matrine (1.0 mg/mL) for 48 h, and fixed with cold 4% paraformaldehyde. After blocking with 5% BSA (Ameresco, USA), the cells were incubated overnight with primary antibodies against KLF4(1:200, CST), TCF4 (1:200, CST) at 4°C. Next, the cells were incubated with Rhodamine Red or Alexa Fluor 488 goat anti-rabbit IgG (1:500, Bioworld Technology, Inc). After counterstaining with 4-6-diamidino-2-phenylindole (DAPI) (Sigma), the slides were observed under a confocal microscope (Zeiss).
Real-time quantitative RT-PCR
Total RNA was extracted using TRIzol reagent (Invitrogen/Life Technologies, Grand Island, NY, USA). cDNA synthesis was performed using reverse transcription reagents (Invitrogen). Quantitative real-time PCR (qPCR) was performed using the SYBR Green PCR master mix (Applied Biosystems, Waltham, MA) using the procedure of 40 cycles of 95°C for 15 s and 60°C for 1 min followed by thermal denaturation.
Cell migration assay
Migration assays were performed using a 24-well Transwell Chamber (Corning, Corning, NY). After treatment with Matrine for 48 h, 1 × 105 cells in 200 μl serum-free medium (SFM) were harvested and seeded in the top chamber, and 600 μl 10% fetal bovine serum (FBS) culture medium was added to the lower chamber. Cells that had migrated and attached to the lower membrane surface were fixed with 4% paraformaldehyde, stained with crystal violet, and counted.
Wound healing assay
After treatment with different concentrations of Matrine for 48 h, cells were then cultured to 70% confluency in DMEM medium with 1% FBS. The cell monolayers were scratched with a 200 ml pipette tip. They were further incubated with fresh medium for 48 h.
CD133+ cells analysis
After treatment with Matrine (1.0 mg/mL) and/or 5-FU, cells were collected and suspended at 1 × 105cells/mL in PBS, and were stained with an anti-CD133 antibody (Miltenyi Biotech, Teterow, Germany) or isotype control antibody (Mouse IgG1) (Miltenyi Biotech) at 4°C in dark for 10 min. CD133+ cells were detected by flow cytometic analysis.
Measurement of apoptosis
Similar to CD133+ cells detection, cells were incubated with indicated drugs for 48 h. The cells were harvested and resuspended in 400-μl Annexin V-FITC binding buffer. The cells were then stained with 5 μl Annexin-V and PI (BD Biosciences) in dark condition at room temperature and subjected to analysis by flow cytometry (Beckton Dickson, San Jose, CA).
Tumor xenograft study
We used 4–5 weeks old male BALB/c nude mice, they were injected with 2 × 106 A549 cells in the fossa axillaris. The mice received the injection of Matrine (20 mg/kg) and/or 5-FU (20 mg/kg) or PBS intraperitoneally every other day. The animals were sacrificed 4 weeks after the injection of tumor cells, and the harvested tissues from the nude mice were excised and subjected to further analyses. Tumor length and width were measured with a vernier calipers, and tumor volumes were calculated with the formula V = 1/2 (length × width2). Tumor tissues were collected, fixed in 10% formaldehyde, and embedded in paraffin for immunohistochemical analysis performed using the specific affinity-purified polyclonal anti-CCND1 antibody.
Statistical analysis
All data are presented as the mean ± SD of three independent experiments. Statistical significance was determined using Student’s two-tailed t test for two groups, one-way ANOVA for multiple groups. P values less than 0.05 were considered statistically significant.
Results
Matrine suppression of symmetric division and self-renewal of NSCLC stem cells
Cancer stem cells tended to divide symmetrically to obtain strong self-renewal abilities. Matrine inhibited Symmetric cell division in stem cells of NSCLC, as presented in immunofluorescence assay (Figure 1(a)), which consequently inhibited the stem cells’ renewal (Figure 1(b)). In the meantime, Matrine significantly decreased signatures markers of stem cell (Figure 1(c)). Since the Wnt pathway is strongly activated in the lung CSCs, and we therefore analyzed whether Matrine has a certain inhibitory effect on it. Figure 1(c) indicated that Matrine blocks the activity of the Wnt pathway.
Figure 1.
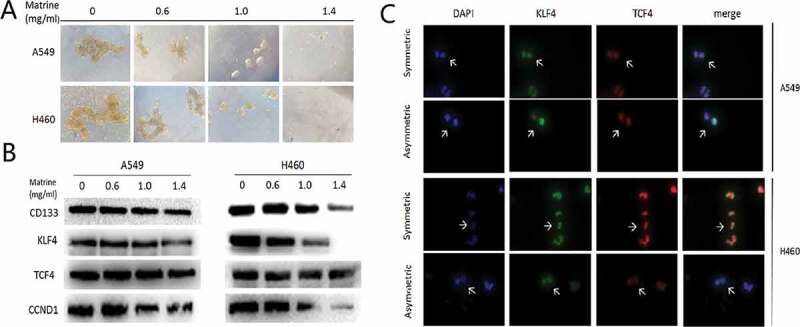
Matrine suppresses lung CSCs properties
(a) The types of division is defined in Matrine-treated and not treated cells and is represented by white arrows. CSCs marker KLF4 and TCF4 were stained to illustrate symmetric division and asymmetric division of lung CSCs.(b) A549 and H460 sphere-forming cells were treated with various concentrations of Matrine in 0.6 mg/ml, 1.0 mg/ml, 1.4 mg/ml for 10 days (scale bar: 100 μm).(c) The protein levels of lung CSCs markers and key components of the Wnt signaling pathway were determined by Western blotting.
Matrine suppresses Wnt pathway and increases the expression of Let-7b in lung CSCs
Wnt pathway is the key canonical pathway in the maintenance of CSCs abilities. In our study, we found that both CCND1 and TCF4 were upregulated in A549 tumor spheres (Figure 2(a)), indicating that the Wnt pathway was activated in lung CSCs. We can also observe a similar phenomenon in CCND1 and TCF4 mRNA expression (Figure 2(b)).
Figure 2.
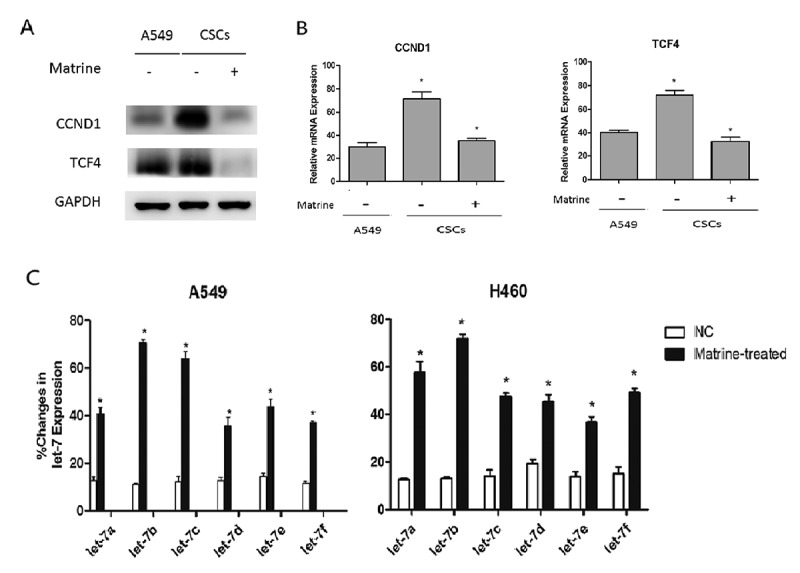
Matrine inhibits Wnt pathway and increases Let-7b expression in lung CSCs
(a, b) A549 CSCs cells were treated with Matrine at a concentration of 1.0 mg/ml for 48 h. (a) Then the expression of CCND1 and TCF4 in A549 cell lines and CSCs was detected by immunoblotting.(b) CCND1 and TCF4 mRNA level detection was also performed by qRT-PCR.(c) The effects of Matrine on Let-7 family expression in lung CSCs were determined by quantitative real-time PCR.
The expression of microRNA changes under various stimuli or drugs, such as hypoxia and chemotherapy, will probably in turn affect the growth of tumor cells. Therefore, we investigated whether the expression of CCND1 was regulated by some miRNAs under the effect of Matrine, because several miRNAs were thought to be the key regulatory factors in the CCND1/Wnt pathway. MiRNAs of the Let-7 family affected the activity of several pathways. It is considered to be an important tumor suppressor which can be evidenced at http://starbase.sysu.edu.cn/index.php (FigureS3) [19]. We can find that most of Let-7 miRNA levels decreased in lung adenocarcinoma (LUAD) and lung squamous carcinoma (LUSC) except for let-7d in LUSC (FigureS3H). Then we observed the changes of miRNAs expression of let7 family in lung CSCs after Matrine therapy using qRT-PCR, and the results showed that the expression of Let-7b increased most significantly among all Let-7 miRNA family after Matrine treatment. Consequently, we focused on Let-7b in the following studies (Figure 2(c)).
Matrine regulates CSCs renewal of NSCLC in a CCND1/WNT signaling dependent way
Since CCND1 is an important fate determiner of CSCs and Matrine inhibited lung CSCs’ expansion, we then examined the expression of CSCs markers of KLF4 and CD133, and found that Matrine, Let-7b, and CCND1 siRNA are all suppressive for CSCs characteristics. Next we studied the role of a possible cascade of Matrine/Let-7b/CCND1 signaling in the formation of CSCs in NSCLC. The spheroid formation result was basically consistent with what showed in Western Blot (Figure 3(a,b)). Then we used CD133 antibody for flow cytometry analysis, it was found that CD133 cells were enriched by CCND1/Wnt signal activator, which could be partially blocked by either Matrine or Let-7b (Figure 3(d,e)). Furthermore, we treated A549 and H460 cells with CCND1 upregulation inducer Tetracycline (TET) and Matrine, respectively, following the combination of which and we found that enforcing CCND1 could abolish the Matrine inhibitory effect (Figure 3(c)). This result evidenced that Let-7b induced downregulation of CCND1 was necessary for Matrine suppression of the self-renewal capacity of NSCLC stem cells.
Figure 3.
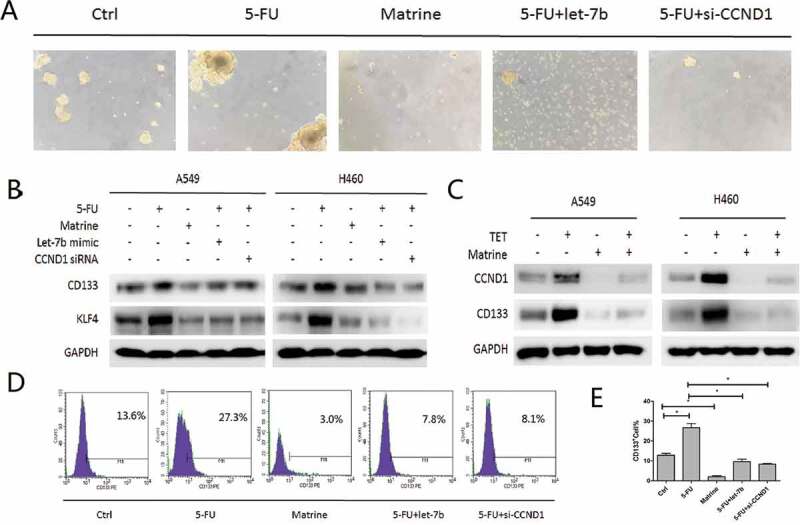
Matrine/Let-7b/CCND1 suppresses CSCs in NSCLC
(a, b) A549 and H460 sphere-forming cells were treated with 5-FU and Matrine alone, or with Let-7b mimic or CCND1siRNA combination.(a) Representative pictures of Spheroids after 10 days of treatment accordingly.(b) CSCs biomarkers CD133 and KLF4 expressions were evaluated with immunoblotting.(c) A549 and H460 cells were treated with CCND1 upregulation inducer Tetracycline (TET) and Matrine alone or in combination.(d, e) Flow cytometric analysis of CD133-positive cells in A549 spheres-forming cells. Columns, mean (n = 3); bars, SD; *, p < 0.05
The upregulation of Let-7 expression is critical to the regulation of CCND1/Wnt
Next, we studied whether inhibition of Let-7 expression could rescue Matrine-induced downregulation of CCND1 protein and other major parts of the Wnt pathway. We treated A549 cells with Matrine, Let-7b mimic, or Matrine in the presence of Let-7b inhibitor for 48 hours. We found that both (Sulforaphane) SFNand Let-7b mimic the repressed expression of CCND1 and (Transcription Factor 4)TCF4. Importantly, the inhibitory effect of Matrine on CCND1 and TCF4 was obviously compromised in the presence of miR-inhibitor (Figure 4(a)). The authenticity of the Matrine/Let-7b/CCND1 pathway in the mRNA level was further validated by performing qRT-PCR (Figure 4(b)).
Figure 4.
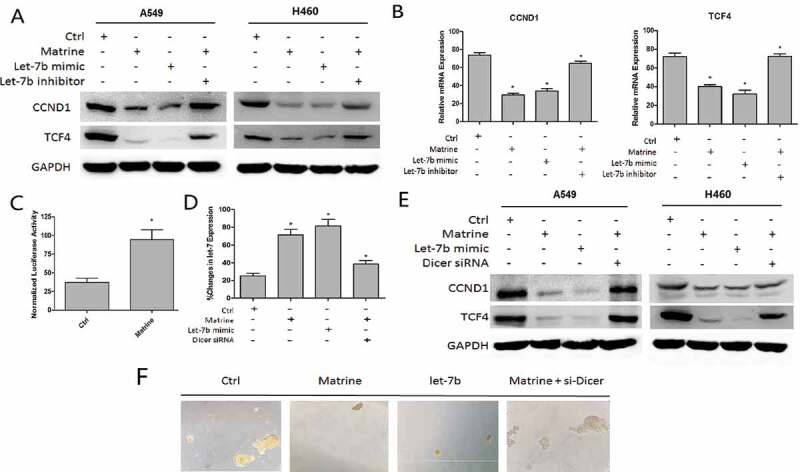
Matrine increased Let-7b level in DICER1-dependent manner
(a–c) A549 and H460 cells were treated with Matrine, Let-7b mimic, or Matrine in the presence of Let-7b inhibitor for 48 h.(a) CCND1 and TCF4 protein levels were determined by immunoblotting.(b) mRNA expression of CCND1 and TCF4 was detected with quantitative real-time PCR.(c) The activity of DICER promoter in A549 cells after being treated with Matrine was determined with Luciferase reporter assay.(d–f) A549 and H460 sphere-forming cells were treated with Matrine in the presence or absence with Dicer 1 siRNA.(d) qPT-PCR was performed to examine the expression of Let-7b. (e) CCND1 and TCF4 protein level was detected by Western blotting.(f) Representative pictures of spheroids after being treated accordingly.
Matrine increased Let-7b level in DICER1 dependent manner
As we studied before, the endoribonuclease DICER1 is a key regulator of Let-7 through sequence-specific silencing, thus promoting the maturation of it. We evaluated whether Matrine increased Let-7b level in DICER1 dependent manner. And the Luciferase reporter assay proved that the activity of DICER promoter in A549 cells increased significantly after being treated with Matrine (Figure 4(c)). Next, we perform qRT-PCR to examine if the expression of Let-7b could change in presence or absence with Dicer 1 siRNA after Matrine treatment. As expected, DICER siRNA could rescue part of the effect of Matrine on Let-7b (Figure 4(d)). The immunoblotting and tumorspheres also revealed the same rules (Figure 4(e,f)).
Matrine increases chemotherapeutic drug-induced toxicity in A549 cells
As shown in Figure 5, Matrine and 5-FU decreased the cell survival rate of A549 cells by 45% and 40%, respectively, while the combination of Matrine and 5-FU reduced the cell survival rate more effectively than the single application. Furthermore, we found that the knockdown of CCND1 enhanced the inhibitory effect of 5-FU, which was consistent with the upregulation of Let-7brestores the cell sensitivity to 5-FU (Figure 5(a)).
Figure 5.
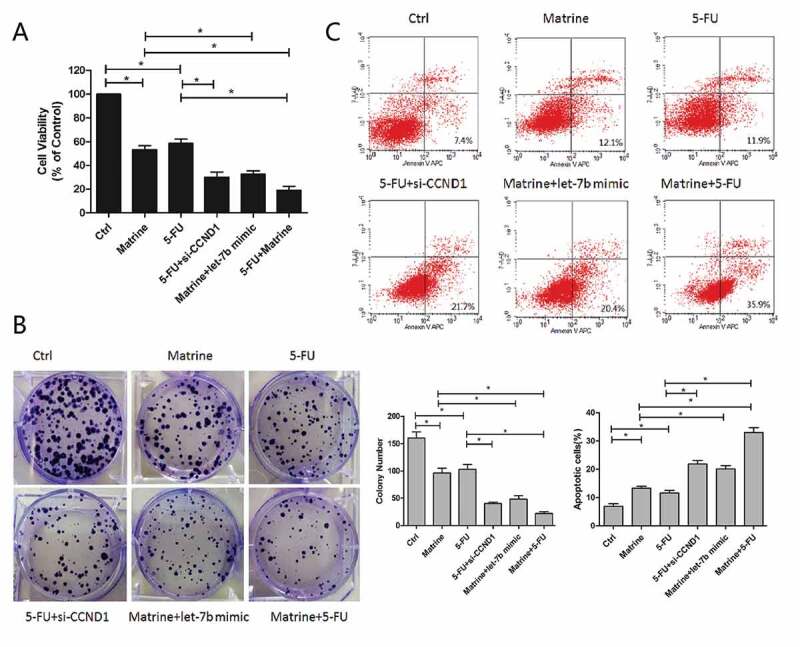
Matrine increases chemotherapeutic drug-induced toxicity of 5-FU in A549 cells
(a) Cell survival rate of A549 was detected after treatment with Matrine and 5-FU alone, or along with Let-7b mimic, CCND1 siRNA, and their combination.(b) A549 cells were plated at low density in 6-well plates, and then cells were treated with Matrine, 5-FU, Let-7b mimic, CCND1 siRNA, or their combination. Columns, mean (n = 3); bars, SD; *, p < 0.05. (c) The ability of combined therapy of Matrine and 5-FU to induce apoptosis was detected by flow cytometry with propidium iodide and Annexin V APC double staining. Columns, mean (n = 3); bars, SD; *, p < 0.05
Similarly, the combination of Matrine and 5-FU significantly reduced colony formation compared with a single reagent (Figure 5(b)).The ability of combined therapy to induce apoptosis was detected by flow cytometry. The results showed that Matrine combined with 5-FU significantly increased cell apoptosis compared with monotherapy, and CCND1 siRNA can enhance the suppressive effect of 5-FU as well (Figure 5(c)).
Matrine repressed tumor proliferation in vivo
In order to whether Matrinecould enhances the sensitivity of NSCLC cells to chemotherapy or not, we carried out experiment in the nude mice xenograft model. A549 cells were implanted into the flanks of mice by subcutaneous injection. Vehicle, Matrine (20 mg/kg), 5-FU (20 mg/kg) or two drugs were intraperitoneally administrated every other day for 4 weeks. The results demonstrated that both Matrine and 5-FU significantly suppressed tumor growth reflected by a decrease of tumor volume and weight, while the combination of Matrine and 5-FU further enhanced the antitumor effect compared with single therapy (Figure 6(a–c)).
Figure 6.
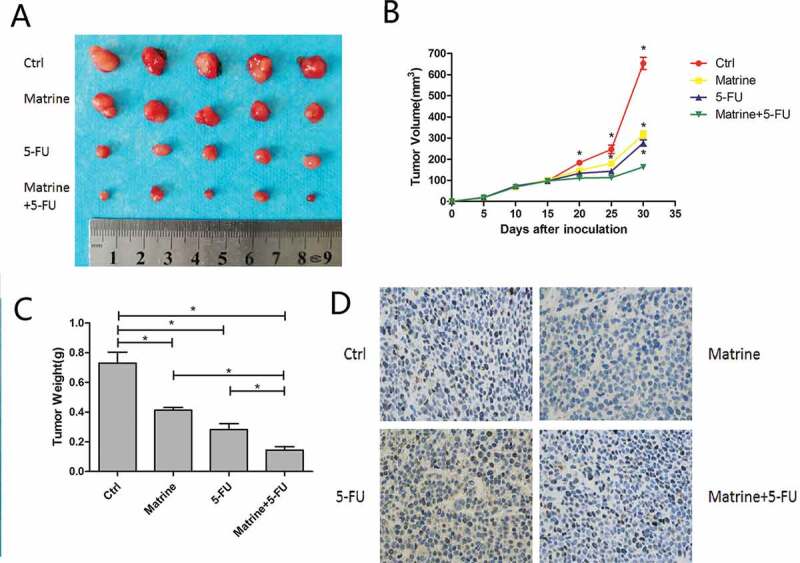
Matrine repressed tumor proliferation in vivo
(a) Cells were subcutaneous injected in nude mice, which intraperitoneally administrated by vehicle, Matrine (20 mg/kg), 5-FU (20 mg/kg), or two drugs.(b) Tumor volume (mm3) was measured at every 5 treatment days.(c) Tumor weights were determined after the treatment was completed on day 30. Both Matrine and 5-FU significantly suppressed tumor volume and weight, while the combination therapy further enhanced the antitumor effect compared with a single therapy. Columns, mean (n = 3); bars, SD; *, p < 0.05(d) In the immunohistochemical analysis of tumor sections, Matrine could effectively inhibit CCND1 expression which was accumulated by 5-FU.
In order to investigate the effect of Matrine on the expression of CCND1 in vivo, anti-CCND1 antibody was used to immunohistochemical analysis of tumor sections. Matrine could effectively inhibit CCND1 expression which was accumulated by 5-FU (Figure 6(d)), further supporting the theory that Matrine functioned through CCND1 deregulation.
Matrine inhibits the cell proliferation and colony formation ability of NSCLCs
The growth ratios of lung cancer cell lines of A549 and H460 cells in different groups were universally inhibited, as determined by MTT assay (FigureS1B), when cells were treated with Matrine at 0.6 mg/ml, 1.0 mg/ml,1.4 mg/ml for 24 h, 48 h, and 72 h, respectively. Notably, Matrine inhibited cell growth was in a dose-dependent manner (FigureS1C and S1D).
Matrine regulates EMT of NSCLC through CCND1/WNT pathway
Evidences have demonstrated the key role of CCND1/Wnt signaling in EMT (Figure S2). In order to investigate the effect of Matrine on CCND1/Wnt mediated EMT, we measured the effect of Matrine on the migration ability of NSCLC cells and the expression of EMT markers. Based on the Transwell cell migration and wound healing test, we found that Matrine could reduce the movement ability of NSCLC cells (FigureS2A, S2B, and S2C) Matrine alleviated EMT by increasing the expression of E-cadherin and decreasing the level of N-cadherin and Vimentin protein (FigureS2D). Let-7b overexpression and CCND1 gene knockout of NSCLC cells also reduced EMT. In summary, these results suggest that Let-7b-mediated inhibition of CCND1/Wnt signaling by Matrine reduces EMT in NSCLC cells.
Discussion
Established evidence suggests that CSCs play a crucial role in the progression, metastasis, and recurrence of human cancers [20]. The mechanisms include unlimited proliferation, tumor self-renewal, heterogeneous progeny, and chemotherapeutic resistance [20–22]. Accordingly, targeting CSCs may provide an effective anti-cancer strategy to overcome drug resistance and reduce recurrence. In recent years, the potential of natural products of medicinal plants of traditional Chinese medicine has been recognized, several natural products and their derivatives belong to the standard series of chemotherapeutic drugs for cancer [23,24]. Matrine is a highly polar basic compound and used in the treatment of hepatitis and hepatic fibrosis and various cancer, including hepatocellular carcinoma, gastric cancer, esophageal cancer, myeloid leukemia, melanoma, breast, and lung cancers [25–30]. Clinical studies have shown that the combination of standard therapy with Matrine can greatly improve the quality of life and immune function of cancer patients [31]. Up until now, the mechanism underlying the anticancer effects of Matrine has rarely been investigated in human NSCLC CSCs.
In the view that p53 and cyclinD 1 interact at multiple levels, while let-7 inhibits the expression of cyclinD 1 [32], and Wnt signaling plays crucial roles in CSC renewal and in the EMT of NSCLC cells [33,34], we hypothesized that let-7 may regulate the self-renewal of NSCLC CSCs by interacting with CCND1/Wnt in the existence of Matrine.
In this study, we found that the expression of Let-7b among the let-7 families increased most significantly after being treated with appropriate concentration of Matrine, then elucidated the key role of Let-7b in regulating lung CSC traits and the key role of the Let-7b/CCND1 axis in Matrine interfering of lung CSCs. The results showed that 5-FU treatment could promote the accumulation of CCND1, which could be effectively inhibited by co-treatment with Matrine and the abnormal expression of Let-7b. Matrine/Let-7b inhibits CSC properties and enhances cytotoxicity induced by chemotherapeutic drugs. Importantly, Matrine sensitizes NSCLC cells to the in vivo effects of 5-Fu, which is related to the inhibition of CCND1 accumulation in 5-Fu-induced tumor tissues.
In light of the results of this study, we found that the expression of Let-7b was up-regulated in lung CSCs, which in turn targeted Wnt/CCND1 pathway. Accordingly, Matrine inhibited CSC properties and enhanced the therapeutic efficacy of 5-FU in NSCLC. These new findings provide new insights into the molecular mechanisms of lung CSCs regulation of Matrine and/or combined with 5-FU.
Supplementary Material
Acknowledgments
The authors acknowledge assistants in the Center for Translational Medicine of First Affiliated Hospital of Xi’an Jiaotong University, for their technical assistance.
Funding Statement
This experiment was supported by National Science Foundation for Young Scientists of China, grant No. 81602597 (Referred to Xin Sun), Foundation Research Project of Shaanxi Province (The Natural Science Fund No. 2018JM7017 (Referred to Xin Sun).
Disclosure statement
Authors declare each has approved this article to be published and that this research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Chen X, Zhi X, Pan P, et al. Matrine prevents bone loss in ovariectomized mice by inhibiting RANKL-induced osteoclastogenesis. Faseb J. 2017;31:4855–4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Liao XZ, Tao LT, Liu JH, et al. Matrine combined with cisplatin synergistically inhibited urothelial bladder cancer cells via down-regulating VEGF/PI3K/Akt signaling pathway. Cancer Cell Int. 2017;17:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhang YB, Luo D, Yang L, et al. Matrine-type alkaloids from the roots of sophora flavescens and their antiviral activities against the hepatitis B virus. J Nat Prod. 2018;81(10):2259–2265. [DOI] [PubMed] [Google Scholar]
- [4].An Q, Han C, Zhou Y, et al. Matrine induces cell cycle arrest and apoptosis with recovery of the expression of miR-126 in the A549 non-small cell lung cancer cell line. Mol Med Rep. 2016;14:4042–4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gu YY, Chen MH, May BH, et al. Matrine induces apoptosis in multiple colorectal cancer cell lines in vitro and inhibits tumour growth with minimum side effects in vivo via Bcl-2 and caspase-3. Phytomedicine. 2018;51:214–225. [DOI] [PubMed] [Google Scholar]
- [6].Rashid HU, Xu Y, Muhammad Y, et al. Research advances on anticancer activities of matrine and its derivatives: an updated overview. Eur J Med Chem. 2019;161:205–238. [DOI] [PubMed] [Google Scholar]
- [7].Huang H, Wang Q, Du T, et al. Matrine inhibits the progression of prostate cancer by promoting expression of GADD45B. Prostate. 2018;78:327–335. [DOI] [PubMed] [Google Scholar]
- [8].Huang M, Xin W.. Matrine inhibiting pancreatic cells epithelial-mesenchymal transition and invasion through ROS/NF-kappaB/MMPs pathway. Life Sci. 2018;192:55–61. [DOI] [PubMed] [Google Scholar]
- [9].Yao Y, Zhao M, Yuan D, et al. Elevated pretreatment serum globulin albumin ratio predicts poor prognosis for advanced non-small cell lung cancer patients. J Thorac Dis. 2014;6:1261–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Barta JA, Powell CA, Wisnivesky JP.. Global epidemiology of lung cancer. Ann Glob Health. 2019;85(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Du L, Morgensztern D. Chemotherapy for advanced-stage non-small cell lung cancer. Cancer J. 2015;21:366–370. [DOI] [PubMed] [Google Scholar]
- [12].Roush S, Slack FJ. The let-7 family of microRNAs. Trends Cell Biol. 2008;18:505–516. [DOI] [PubMed] [Google Scholar]
- [13].Wang M, Li Y, Xiao G-D, et al. H19 regulation of oestrogen induction of symmetric division is achieved by antagonizing Let-7c in breast cancer stem-like cells. Cell Prolif. 2019;52:e12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36:1461–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xu C, Xiao G, Zhang B, et al. CCAT1 stimulation of the symmetric division of NSCLC stem cells through activation of the Wnt signalling cascade. Gene Ther. 2018;25:4. [DOI] [PubMed] [Google Scholar]
- [16].Sun X, Xu C, Tang SC, et al. Let-7c blocks estrogen-activated Wnt signaling in induction of self-renewal of breast cancer stem cells. Cancer Gene Ther. 2016;23:83. [DOI] [PubMed] [Google Scholar]
- [17].Czerwonka A, Kalawaj K, Slawinska-Brych A, et al. Anticancer effect of the water extract of a commercial Spirulina (Arthrospira platensis) product on the human lung cancer A549 cell line. Biomed Pharmacothe. 2018;106:292–302. [DOI] [PubMed] [Google Scholar]
- [18].Cruz-Lozano M, Gonzalez-Gonzalez A, Marchal JA, et al. Hydroxytyrosol inhibits cancer stem cells and the metastatic capacity of triple-negative breast cancer cell lines by the simultaneous targeting of epithelial-to-mesenchymal transition, Wnt/beta-catenin and TGFbeta signaling pathways. 2018. [DOI] [PubMed]
- [19].Li J-H, Liu S, Zhou H, et al. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein–RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42:D1, D92–D97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sancho P, Barneda D, Heeschen C. Hallmarks of cancer stem cell metabolism. Br J Cancer. 2016;114:1305–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Binju M, Padilla MA, Singomat T, et al. Mechanisms underlying acquired platinum resistance in high grade serous ovarian cancer - a mini review. Biochim Biophy Acta. 2018;1863:371–378. [DOI] [PubMed] [Google Scholar]
- [22].Steinbichler TB, Dudas J, Skvortsov S, et al. Therapy resistance mediated by cancer stem cells. Seminars in cancer biology. 2018. [DOI] [PubMed]
- [23].Li X, Li X, Huang N, et al. A comprehensive review and perspectives on pharmacology and toxicology of saikosaponins. Phytomedicine. 2018;50:73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Liao YH, Li CI, Lin CC, et al. Traditional Chinese medicine as adjunctive therapy improves the long-term survival of lung cancer patients. J Cancer Res Clin Oncol 2017;143:2425–2435. [DOI] [PubMed] [Google Scholar]
- [25].Guo S, Chen Y. Matrine is a novel inhibitor of the TMEM16A chloride channel with antilung adenocarcinoma effects. 2018. [DOI] [PubMed]
- [26].Jin B, Wang W, Meng XX, et al. Let-7 inhibits self-renewal of hepatocellular cancer stem-like cells through regulating the epithelial-mesenchymal transition and the Wnt signaling pathway. BMC Cancer. 2016;16:863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sun X, Zhuo XB, Hu YP, et al. A novel matrine derivative WM622 inhibits hepatocellular carcinoma by inhibiting PI3K/AKT signaling pathways. Mol Cell Biochem. 2018;449:47–54. [DOI] [PubMed] [Google Scholar]
- [28].Wu J, Hu G, Dong Y, et al. Matrine induces Akt/mTOR signalling inhibition-mediated autophagy and apoptosis in acute myeloid leukaemia cells. J Cell Mol Med. 2017;21:1171–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang JW, Su K, Shi WT, et al. Matrine inhibits the adhesion and migration of BCG823 gastric cancer cells by affecting the structure and function of the vasodilator-stimulated phosphoprotein (VASP). Acta Pharmacol Sin. 2013;34:1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Feng Y, Ying HY, Qu Y, et al. Novel matrine derivative MD-1 attenuates hepatic fibrosis by inhibiting EGFR activation of hepatic stellate cells. Protein Cell. 2016;7:662–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gao L, Wang KX, Zhou YZ, et al. Uncovering the anticancer mechanism of compound kushen injection against HCC by integrating quantitative analysis, network analysis and experimental validation. Sci Rep. 2018;8:624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sun X, Tang SC, Xu C, et al. DICER1 regulated let-7 expression levels in p53-induced cancer repression requires cyclin D1. J Cell Mol Med. 2015;19:1357–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chandimali N, Huynh DL. MicroRNA-122 negatively associates with peroxiredoxin-II expression in human gefitinib-resistant lung cancer stem cells. Cancer Gene Ther. 2019. Sep; 26(9–10):292-304. doi: 10.1038/s41417-018-0050-1 [DOI] [PMC free article] [PubMed]
- [34].Huang WC, Kuo KT, Adebayo BO, et al. Garcinol inhibits cancer stem cell-like phenotype via suppression of the Wnt/beta-catenin/STAT3 axis signalling pathway in human non-small cell lung carcinomas. J Nutr Biochem. 2018;54:140–150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


