Figure 7.
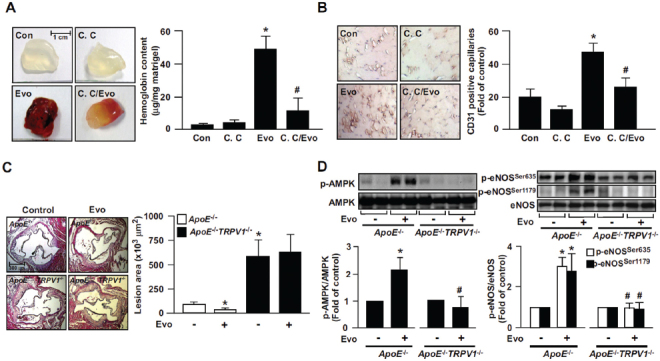
Role of AMPK in evodiamine-induced angiogenesis in WT mice and retardation of atherosclerosis in ApoE−/− mice. (A) Eight-week-old male WT mice were subcutaneously injected with matrigel plugs containing heparin (50 U/mL) with or without evodiamine (1 µmol/L) or compound C (10 µmol/L). At 7 d postadministration, plugs were removed and photographed, and the hemoglobin content was analyzed. Data are mean ± SD from eight mice. *P < 0.05 versus WT mice without evodiamine treatment, #P < 0.05 versus WT mice with evodiamine treatment. (B) Immunohistochemical staining of ischemic tissues with anti-CD31 antibody and quantitative analysis of capillary density in mice on postoperative d 14. Capillary density was expressed as the number of capillaries per field (200×). Hematoxylin was used as counterstaining. Data are mean ± SD from five mice. *P < 0.05 versus WT mice without evodiamine treatment, #P < 0.05 versus WT mice with evodiamine treatment. (C, D) Male ApoE−/− and ApoE−/−TRPV1−/− mice at 4 months received daily oral administration of evodiamine (10 mg/kg body weight) or saline (vehicle control) by gastric gavage for 4 wks, and hearts and aortas were harvested. The atherosclerotic lesions that developed at the aortic roots were quantitated. Tissue extracts from aortas were immunoblotted with phosphorylated AMPK at Thr172, AMPK, phosphorylated eNOS at Ser635 and Ser1179, and eNOS. Data are mean ± SD from 10 mice. *P < 0.05 versus the ApoE−/− mice control group, #P < 0.05 versus ApoE−/− mice with evodiamine treatment.
