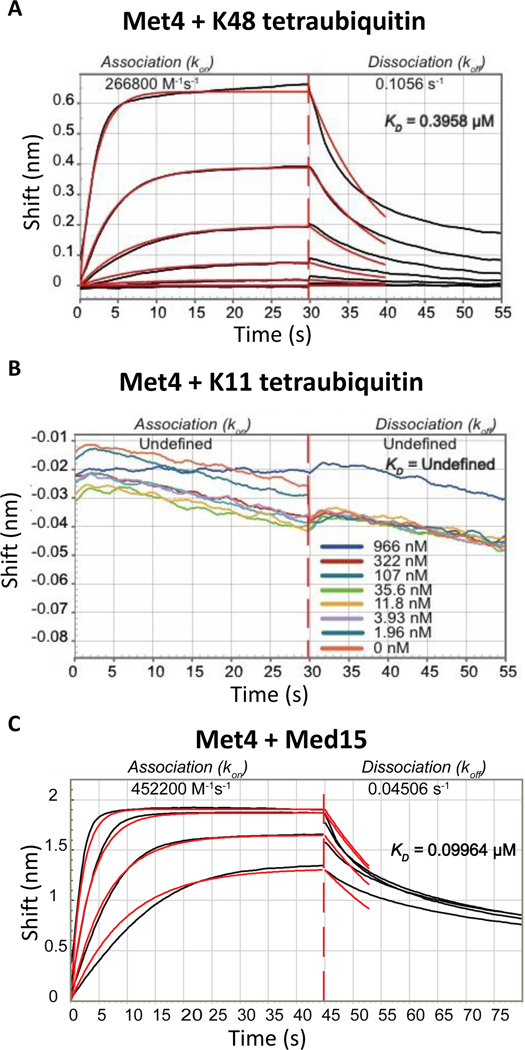
Figure 5. The tandem ubiquitin binding domain in Met4 is selective for K48 ubiquitin chains and interacts with the mediator component Med15.
(A) Biolayer interferometry with sensor immobilized recombinant Met4 (residues 76 to 160) and K48-linked tetraubiquitin. Concentrations used were: 1450, 483, 161, 53, 17, and 5.6 nM.
(B) Same as panel A, but K11-linked tetraubiquitin was used as the ligand. No detectable binding was observed.
(C) Same as panel B, but interaction with the mediator complex Med15 (residues Med15 1–651 Δ239–271, Δ373–483) was analyzed.