Abstract
Purpose of Review
The present review aimed to explore the range and characteristics of interventions that utilize WAM and descriptively summarize the efficacy of these interventions.
Recent Findings
A total of 65 articles (61 studies) were included in this review. Most of the WAM-based interventions (n=58) were designed to improve physical activity (PA). Interventions targeting sedentary behavior (SB) were much less common (n=12), and even less frequent were WAM-based sleep interventions (n=3). Most studies tested the feasibility of WAM-based interventions; hence, efficacy of these interventions in improving PA, SB, and/or sleep could not be conclusively determined. Nonetheless, WAM-based interventions showed considerable potential in increasing PA and decreasing SB.
Summary
WAM-based PA interventions exhibited preliminary efficacy in increasing PA. Although not as many interventions were focused on SB, current interventions also showed potential in decreasing sedentary time. Meanwhile, more evidence is needed to determine the utility of WAM in improving sleep. Major challenges with including WAM as part of interventions are reduced engagement in using the devices over time and the rapid changes in technology resulting in devices becoming obsolete soon after completion of an efficacy trial.
Keywords: wearable activity monitors, fitness trackers, physical activity, sedentary behavior, sleep
Introduction
The market for wearable activity monitors (WAM), devices worn to track physical activity (PA), sleep, and other movement-based behaviors [1], has seen exponential growth over recent years. According to the International Data Corporation, 34.2 million units were sold during the second quarter of 2019 alone [2]. Similarly, a nationwide survey of US adults reported that 12.5% of its respondents were current users of WAM [3]. Advances in sensor technology and improvements in the algorithms used to analyze sensor data have made WAM more accurate [4]. The popularity of these wearable devices, coupled with their increasing capabilities, lend themselves well to clinical research.
Several systematic reviews have examined the validity and reliability of WAM in terms of measuring PA and sleep [1, 5–8]. Overall, WAM were accurate and reliable at counting steps and measuring activity duration [1, 5, 6]. In terms of sleep, WAM were highly correlated with polysomnography in measuring total sleep time [7]. In addition to validation studies, feasibility studies have shown that WAM were generally well-accepted by the intended users [9, 10].
Given the improvements in the validity and reliability of WAM and their acceptability across a wide range of users, it is not surprising that the use of WAM in clinical trials is increasing [1]. However, the majority of these studies utilized WAM as a more convenient and cost-effective alternative to research-grade instruments to collect data [4]. The effect of WAM as the intervention or as a supplement to the intervention are largely unknown. Therefore, the purpose of this scoping review is to examine the effect of WAM as the main intervention or as a supplement to the intervention on PA, sedentary behavior (SB), and/or sleep. Specifically, this review (1) explores the range (e.g., study population, target condition) and characteristics of interventions that utilized WAM, and (2) descriptively summarizes the preliminary efficacy of these interventions.
Methods
This review was conducted following the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) guidelines [11].
Search Strategy
PubMed, CINAHL Plus, and Embase were systematically searched for relevant studies with the guidance of a reference librarian. The database search was conducted in October 2019. Full search strategies for each database are described in the Online Resource 1.
Study Selection
After eliminating duplicates, articles were screened in a 3-step process: by title, by abstract, and by full text. Articles were included if they met the following eligibility criteria: (1) used a randomized-controlled trial or quasi-experimental design, (2) tested an intervention using direct-to-consumer WAM (by itself or as a component of the study intervention), (3) had a measure of PA, SB, or sleep as an outcome variable, (4) was published between January 2009 and September 2019, and (5) was written in English. An article was excluded if it: (1) was only available as an abstract, (2) was only a study protocol, (3) used only a mobile application (app), (4) used only a pedometer, or (5) used only a WAM to evaluate the intervention.
The primary author reviewed the titles and abstracts. At least two independent reviewers assessed the full texts for inclusion (MIC/CCI/CEK/EJB). In case of disagreement regarding the inclusion of a study, a third person (LEB) decided whether the study was included in the scoping review.
Data Charting
To aid in summarizing the findings, information on study characteristics (i.e., authors, year published, country, study design, study/intervention duration, target population, sample size, outcome variables); device characteristics (i.e., brand, model, location worn, activities measured); intervention characteristics (i.e., components, control condition); and study outcomes pertaining to PA, SB, and sleep were collected using an electronic data abstraction form. The primary author abstracted the data, which were then checked by another author (CCI/CEK/LEB) for accuracy.
Results
Search Result
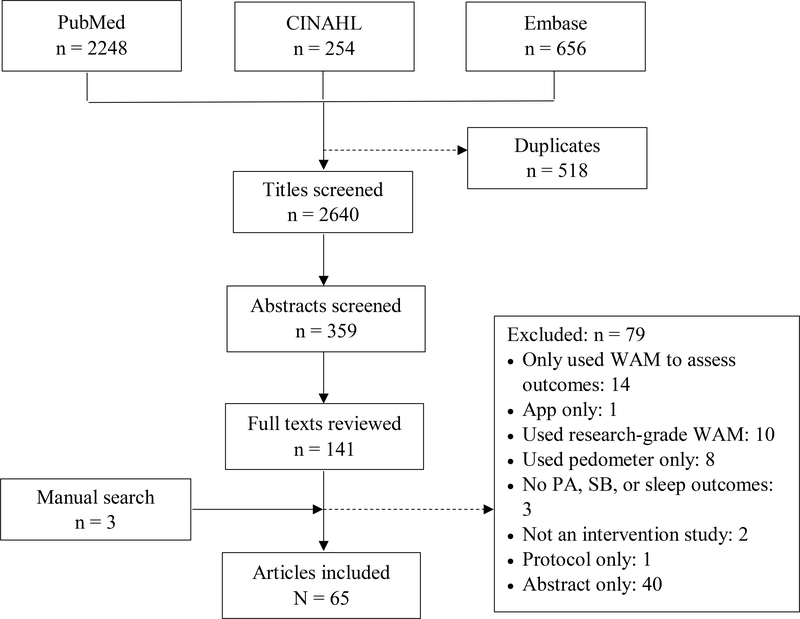
A total of 65 articles, representing 61 studies—one article reported the findings from two studies [12] and 5 studies were used for multiple articles (10 in total) [13–22]—were included in this scoping review (Figure 1).
Figure 1.
Diagram of search and screening process. Acronyms: wearable activity monitor (WAM), physical activity (PA), sedentary behavior (SB)
Study Characteristics
The majority of the studies were conducted in the United States (n=40); 8 were conducted in Canada, 6 in Australia, 3 each in Singapore and South Korea, and one each in Germany and the Netherlands. Most of the interventions targeted PA (n=45), followed by interventions targeting both PA and SB (n=11), PA and sleep (n=2), SB (n=1), sleep (n=1), and only 1 targeted all 3 behaviors. Thirty-four of the studies used a randomized controlled trial design and 27 were quasi-experimental. Study duration ranged from 2 weeks to 12 months, while intervention duration ranged from one week to 10 months. The majority of the studies (n=50) recruited adult participants. Twenty-nine studies targeted populations with a health condition that could impact and/or was impacted by PA, SB, and/or sleep (e.g., cancer, arthritis, chronic pain, insomnia). Finally, sample sizes ranged from 10 to 800 participants (median: 53; mean: 99) (Table 1).
Table 1.
Study Characteristics
| Author | Study Design | Duration (Study/Intervention) | Target Population | Sample Size |
|---|---|---|---|---|
| Physical Activity | ||||
| 2015 | ||||
| A Cadmus-Bertram LA et al.[13, 14] | RCT | 16 weeks | Females, post-menopausal overweight, sedentary, | 51 (49 completed) |
| Garde A et al.[33] | Quasi-experimental | 2 weeks / 1 week | Children (8y–13y) | 54 (44 analyzed) |
| Hayes LB and Camp CM[61] | Quasi-experimental | 5 school weeks | Children | 10 (6 analyzed) |
| Martin SS et al.[62] | RCT | 5 weeks / 4 weeks | Adults (18y–69y) | 48 |
| 2016 | ||||
| Choi JW et al.[27] | RCT | 12 weeks | Females, sedentary, pregnant (18y–40y) | 30 (29 completed) |
| B Finkelstein EA et al.[16] | RCT | 12 months / 6 months | Full-time employees (21y–65y) | 800 |
| Garde A et al.[34] | RCT, cross-over | 4 weeks / 1 week | Children (9y–13y) | 42 |
| Hooke MC et al.[44] | Quasi-experimental | 17 days / 5 days | Children with acute lymphoblastic leukemia (6y–18y) | 17 (16 completed) |
| Le A et al.[63] | Quasi-experimental | ∼7 months / 6 months | Pediatric cancer survivor (>=15y) | 19 (15 completed) |
| Poirier J et al.[25] | RCT | 7 weeks / 6 weeks | Employees | 265 (217 completed) |
| 2017 | ||||
| Abrantes AM et al.[23] | Quasi-experimental | 12 weeks | Females with alcohol use disorder and depressive symptoms (18y–65y) | 20 |
| C Adams MA et al.[30] | RCT | 4 months | Adults, overweight/obese, sedentary | 96 |
| Chung AE et al.[36] | Quasi-experimental | 2 months | College students | 12 |
| Evans EW et al.[12] | Quasi-experimental | Study 1: 4 weeks Study 2: 6 weeks |
5th and 6th graders | Study 1: 32 Study 2: 42 |
| Gell NM et al.[45] | Quasi-experimental | 4 weeks | Cancer survivors | 26 (24 completed) |
| D Losina E et al.[20] | Quasi-experimental | 26 weeks / 24 weeks | Adults, sedentary | 300 (292 analyzed) |
| McMahon SK et al.[64] | RCT | 8 months / 8 weeks | Older adults (>=70y) | 102 (100 analyzed) |
| Patel MS et al.[31] | RCT | 26 weeks / 12 weeks | Adult dyads | 206 (200 analyzed) |
| Rote AE[65] | Quasi-experimental | ∼5 months / 10 weeks | College students | 120 (56 analyzed) |
| Shin DW et al.[37] | RCT | 12 weeks | Male college students, overweight/obese(19y–45y) | 105 (98 analyzed) |
| Yeung J et al.[66] | Quasi-experimental | 8 weeks / 4 weeks | Medical/Surgical residents | 86 (26 completed) |
| Zhang XC et al.[67] | Quasi-experimental | 26 weeks | Females with advance ovarian cancer | 10 |
| 2018 | ||||
| Bade BC et al.[68] | Quasi-experimental | 12 weeks | Adults with lung cancer | 35 |
| Chokshi NP et al.[32] | RCT | 24 weeks / 16 weeks | Adults with ischemic heart disease (>=18y) | 105 |
| DiFrancisco J et al.[69] | RCT | 10 months | 1st year medical students (17y–50y) | 120 (113 analyzed) |
| Duscha BD et al.[70] | RCT | 12 weeks | Adults completing cardiac rehab | 32 (25 completed) |
| Gremaud AL et al.[35] | RCT | 10 weeks | Healthy adults with sedentary jobs | 146 (144 analyzed) |
| Hacker ED et al.[50] | Quasi-experimental | ∼8 weeks / 6 weeks | Adults scheduled for hematopoietic stem cell transplantation (>=18y) | 10 |
| Heale LD et al.[71] | Quasi-experimental | 5 weeks | Adolescents with juvenile idiopathic arthritis (12y–18y) | 31 (28 analyzed) |
| Kooiman TJM et al.[72] | RCT | 12 weeks | Adults with type 2 diabetes | 72 (66 analyzed) |
| Liau AK et al.[73] | Quasi-experimental | 5 weeks / 3 weeks | Adults (18y–50y) | 85 |
| McDermott MM et al.[74] | RCT | 9 months | Adults with peripheral artery disease (≥50y) | 200 (198 analyzed) |
| Nyrop KA et al.[75] | Quasi-experimental | 6–12 weeks (during chemotherapy) | Females with early stage breast cancer (21y–64y) | 127 (100 analyzed) |
| Ovans JA et al.[76] | Quasi-experimental | 24 weeks / 12 weeks | Children/adolescents with brain tumors (7y–18y) | 20 (11 completed) |
| Polgreen LA et al.[41] | RCT | 180 days | Adults with diabetes, obesity (19y–75y) | 138 |
| Vandelanotte C et al.[39] | RCT | 3 months | Adults with overweight/obesity | 243 |
| Yoon SM et al.[77] | RCT | 12 months / 6 months | Adults | 79 (73 randomized) |
| 2019 | ||||
| Amorim AB et al.[28] | RCT | 6 months | Adults with chronic lower back pain | 68 (55 completed) |
| Cadmus-Bertram LA et al.[24] | RCT | 12 weeks | Breast/colorectal cancer survivors + support partners | 50 (47 survivors completed) |
| Cheung NW et al.[78] | RCT | 38 weeks | Females who had gestational diabetes | 60 (37 analyzed) |
| Christiansen MB et al.[79] | RCT | 12 months / 6 months | Adults receiving outpatient PT for total knee replacement | 43 (29 completed) |
| Deka P et al.[29] | RCT | 8 weeks | Adults with heart failure | 30 |
| Janevic MR et al.[40] | RCT | 8 weeks / 6 weeks | Older African Americans with chronic pain | 50 (44 completed) |
| D Meints SM et al.[19] | Quasi-experimental | 26 weeks / 24 weeks | Adults, sedentary | 300 (225 analyzed) |
| C Phillips CB et al. | RCT | 4 months | Adults, sedentary with overweight, obesity | 96 |
| Van Blarigan EL et al.[80] | RCT | 84 days | Adults with non-metastatic colon/rectal cancer | 42 (39 analyzed) |
| Physical Activity & Sedentary Behavior | ||||
| 2013 | ||||
| E Barwais FA et al.[38] | RCT | 5 weeks / 4 weeks | Adults, sedentary | 33 |
| 2015 | ||||
| E Barwais FA et al.[22] | Secondary analysis focusing on the IG | 5 weeks / 4 weeks | Adults, sedentary | 20 (18 analyzed) |
| 2017 | ||||
| Gilson ND et al.[49] | Quasi-experimental | 28 weeks / 20 weeks | Male truck drivers | 26 (19 completed) |
| Mendoza JA et al.[81] | RCT | 13 weeks / 10 weeks | Pediatric cancer survivors (14y–18y) | 60 (59 analyzed) |
| 2018 | ||||
| Buchele Harris H and Chen WY[52] | Quasi-experimental | 7 weeks / 4 weeks | 5th graders | 116 |
| Ezeugwu VE et al.[53] | Quasi-experimental | 16 weeks / 8 weeks | Adults discharged from inpatient stroke rehab | 34 |
| Li LC et al.[82] | RCT | 6 months / 2 months | Adults with knee osteoarthritis (≥50y) | 61 |
| Olsen HM et al.[48] | Quasi-experimental | 6 weeks | Employees | 113 (30 completed) |
| Pope ZC et al.[46] | RCT | 10 weeks | Breast cancer survivors (≥21y) | 30 (20 analyzed) |
| 2018 | ||||
| Trinh L et al.[47] | Quasi-experimental | 24 weeks / 12 weeks | Prostate cancer survivors | 46 |
| 2019 | ||||
| Lynch BM et al.[54] | RCT | 24 weeks / 12 weeks | Breast cancer survivors | 83 (72 analyzed) |
| Muellmann S et al.[26] | RCT | 12 weeks / 10 weeks | Older adults (60y–80y) | 589 (405 completed) |
| Sedentary Behavior | ||||
| 2018 | ||||
| Guitar NA et al.[83] | Quasi-experimental | 8 weeks | Employees | 22 |
| B Sloan RA et al. | RCT | 12 months / 6 months | Full-time employees (21–65y) | 800 |
| Sleep | ||||
| 2017 | ||||
| Kang SG et al.[56] | RCT | 4 weeks | Adults with insomnia disorder (18–65y) | 19 |
| Physical Activity & Sleep | ||||
| 2016 | ||||
| Crowley O et al.[43] | Quasi-experimental | 12 months / 9 months | Employees | 565 (510 analyzed) |
| Melton BF et al.[42] | RCT | 14 weeks / 6 weeks | Female African American college students (18–24y) | 69 (50 completed) |
| Physical Activity, Sedentary Behavior & Sleep | ||||
| 2018 | ||||
| Choi JY et al.[55] | Quasi-experimental | 9 weeks | College students, sedentary | 70 (63 analyzed) |
Note:
Two articles on the same study, reporting on different outcomes. RCT – randomized-controlled trial.
Device Characteristics
As of November 2019, all but one of the WAM models included in this review have been discontinued. Two studies needed to use a different WAM after the original WAM was discontinued during the course of the study [23, 24]. The majority of the studies used Fitbit models (n=48), and most of the models were wrist-worn (n=14). Among the different attributes measured, 19 WAM recorded the number of steps taken, 17 calculated calories expended, 16 measured distance traveled, and 11 tracked the number of minutes spent in different PA intensities. Fifteen of the WAM recorded sleep duration and 11 provided a summary of sleep quality. Lastly, 5 of the WAM measured time spent being sedentary.
Intervention Characteristics
Table 2 summarizes the various intervention strategies used in the reviewed studies. All but one study intervention [25] used at least two behavioral strategies, with self-monitoring being the most commonly used strategy (n=60). Self-monitoring was implemented using the WAM’s display (if available) and/or the WAM’s companion app or website. Two studies had a website specially designed for participants to monitor their activity and receive feedback [25, 26]. Similarly, 2 studies developed an app for self-monitoring and delivering feedback [27, 28] and one study provided participants with paper exercise diaries in addition to the WAM’s companion app [29]. Only one study did not provide their participants access to the WAM’s companion app or website to prevent any potential confounding with the intervention [17, 30].
Table 2.
Intervention Characteristics and Study Outcomes
| Author | Goal- Setting | Social Support | Reward | Education/Training | Comparison | Findings |
|---|---|---|---|---|---|---|
| Physical Activity | ||||||
| 2015 | ||||||
| A Cadmus-Bertram LA et al.[13, 14] | Both | -- | -- | -- | ◆ | Step count: Δbetween study groups ↑ MVPA: Δbetween study groups ↑ Light PA: Δbetween study groups ↓ |
| Garde A et al.[33] | -- | Community, Competition | Digital reward | -- | ◆ | Step count: Δbetween study groups ↑ Total PA: Δbetween study groups ↑ |
| Hayes LB and Camp CM[61] | Adaptive | In-person | In-kind | -- | N/A | Step count: Δpre/post-intervention ↑ |
| Martin SS et al.[62] | Static | Text messages | -- | -- | ◆ ◆ | Step count: Δbetween study groups ↑* Total PA: Δbetween study groups ↑* |
| 2016 | ||||||
| Choi JW et al.[27] | Both | App | -- | One-on-one | ◆ | Step count: Δbetween study groups ↑ |
|
B Finkelstein EA et al.[16] B Sloan RA et al.[51] |
Static | -- | Cash/Charitable donation | Printed material | ◆ ◆ ○ | Step count: Δbetween study groups ↑* MVPA: Δbetween study groups ↑* Sedentary time: Δbetween study groups ↑ |
| Garde A et al.[34] | -- | Community, Competition | Digital reward | -- | Not specified | Step count: Δbetween study groups ↑* Total PA: Δbetween study groups ↑* |
| Hooke MC et al.[44] | Both | -- | -- | N/A | Step count: Δpre/post-intervention ↓ | |
| Le A et al.[63] | Static | -- | -- | Not specified | N/A | MVPA: Δpre/post-intervention ↑ |
| Poirier J et al.[25] | Adaptive | Community*, Competition* | Digital reward | -- | ○ | Step count: Δbetween study groups ↑* |
| 2017 | ||||||
| Abrantes AM et al.[23] | Adaptive | Phone | -- | One-on-one | N/A | Step count: ΔPP ↑* MVPA: Δpre/post-intervention ↑ Total PA: Δpre/post-intervention ↑* |
|
C Adams MA et al.[30] C Phillips CB et al.[17] |
Both | Text messages | Cash | Printed material | ◆ ◆ ◆ | Step count: Δbetween study groups ↑* (Adaptive Goal > Static Goal; Immediate Reward > Delayed Reward) MVPA: Δbetween study groups ↑* (IR>DR); Δbetween study groups ↑ (SG>AG) |
| Chung AE et al.[36] | Adaptive | Community, Competition | In-kind | NA | PA goal: only 58% reached daily step goal | |
| Evans EW et al.[12] | Static | -- | In-kind | Printed material | N/A | Step count: Δpre/post-intervention ↑ |
| Static | Competition | Cash, In-kind | Group session | ◆ ○ | Step count: Δbetween study groups ↓ MVPA: Δbetween study groups ↓ |
|
| Gell NM et al.[45] | Adaptive | Phone, Text messages | -- | Printed material, Health coaching | N/A | Step count: Δpre/post-intervention ↓ MVPA: Δpre/post-intervention ↓ |
|
D Losina E et al.[20] D Meints SM et al.[19] |
Adaptive | Community | Cash | -- | N/A | Step count: Δbetween study groups ↑* |
| McMahon SK et al.[64] | Adaptive | Community, Competition | -- | Group session | ◆ ◆ ◆ | Step count: Δbetween study groups ↑ |
| Patel MS et al.[31] | Both | Community | In-kind | -- | ◆ | Step count: Δbetween study groups ↑* |
| Rote AE[65] | -- | -- | -- | -- | ■ ○ | Step count: Δbetween study groups ↑* |
| Shin DW et al.[37] | Static | -- | Cash | One-on-one, Printed material | ■ ■ | Total PA: Δbetween study groups ↑* |
| Yeung J et al.[66] | Static | -- | -- | -- | N/A | Step count: Δpre/post-intervention ↑* |
| Zhang XC et al.[67] | Both | Phone | -- | Video, exercise training | N/A | Step count: Δpre/post-intervention ↑* MVPA: Δpre/post-intervention ↑ Light PA: Δpre/post-intervention ↑ Moderate PA: Δpre/post-intervention ↑* |
| 2018 | ||||||
| Bade BC et al.[68] | Adaptive | Text messages | -- | One-on-one | ◆ | Step count: Δbetween study groups ↑ |
| Chokshi NP et al.[32] | Both | -- | Cash | -- | ◆ | Step count: Δbetween study groups ↑* |
| DiFrancisco J et al.[69] | Both | -- | -- | -- | ◆ ○ | Step count: ΔBET ↑* |
| Duscha BD et al.[70] | Adaptive | Phone, Text messages | -- | Phone | ■ | Step count: Δbetween study groups ↑ MVPA: Δbetween study groups ↑* |
| Gremaud AL et al.[35] | Adaptive* | Competition | Digital reward | -- | ◆ | Step count: Δbetween study groups ↑ Total PA: Δbetween study groups ↑ |
| Hacker ED et al.[50] | Adaptive | -- | -- | One-on-one | N/A | Step count: Δpre/post-intervention ↑ Total PA: Δpre/post-intervention ↓ |
| Heale LD et al.[71] | Adaptive | -- | -- | -- | N/A | MVPA: Δpre/post-intervention ↑ |
| Kooiman TJM et al.[72] | Both | -- | -- | Video, Online | ○ | Step count: IG>CG (post-int only) MVPA: Δbetween study groups ↑* |
| Liau AK et al.[73] | Static | -- | -- | Online | ◆ | Step count: Δbetween study groups ↑* |
| McDermott MM et al.[74] | Both | Phone | -- | Exercise training | ■ | Exercise: Δbetween study groups ↑* |
| Nyrop KA et al.[75] | Static | -- | -- | Printed material | N/A | PA goal: post-int, only 19% achieved goal |
| Ovans JA et al.[76] | Adaptive | -- | -- | One-on-one, Phone | N/A | Step count: Δpre/post-intervention ↑ |
| Polgreen LA et al.[41] | Adaptive | -- | -- | Printed material | ◆ ◆ | Step count: Δbetween study groups ↓ |
| Vandelanotte C et al.[39] | Adaptive | -- | -- | Online | ■ | MVPA: Δbetween study groups ↑* Total PA: Δbetween study groups ↑* Sitting time: Δbetween study groups ↓* |
| Yoon SM et al.[77] | -- | -- | -- | ■ | Exercise: Δbetween study groups ↓* | |
| 2019 | ||||||
| Amorim AB et al.[28] | Adaptive | Phone | -- | Printed material, Health coaching | ■ | Step count: Δbetween study groups ↑ MVPA: Δbetween study groups ↓ Light PA: Δbetween study groups ↑ |
| Cadmus-Bertram LA et al.[24] | Both | Email, Community | -- | One-on-one, Printed material | ■ | Step count: Δbetween study groups ↑* MVPA: Δbetween study groups ↑* |
| Cheung NW et al.[78] | Both | Text messages | -- | One-on-one, Phone | ■ | Step count: IG>CG (post-int only) |
| Christiansen MB et al.[79] | Both | -- | -- | Printed material | ■ | Step count: Δbetween study groups ↑* MVPA: Δbetween study groups ↑* |
| Deka P et al.[29] | Static | Community | -- | Printed material, Online | ◆ | Exercise: Δbetween study groups ↓ |
| Janevic MR et al.[40] | -- | -- | -- | -- | ○W | Walking: Δbetween study groups ↑ |
| Van Blarigan EL et al.[80] | Static | -- | -- | Printed material | ■W | Step count: Δbetween study groups ↑ MVPA: Δbetween study groups ↑ |
| Physical Activity & Sedentary Behavior | ||||||
| 2013 | ||||||
| E Barwais FA et al.[22, 38] | Adaptive | -- | -- | ns | Light PA: Δbetween study groups ↑* Moderate PA: Δbetween study groups ↑* Vigorous PA: Δbetween study groups ↑* Exercise: Δbetween study groups ↑* Sedentary time: Δbetween study groups ↓* |
|
| 2017 | ||||||
| Gilson ND et al.[49] | Adaptive | Community, Competition | Cash | Group session, Printed material | N/A | Total PA: Δpre/post-intervention ↑ Sedentary time: Δpre/post-intervention ↓ |
| Mendoza JA et al.[81] | Both | Text messages, Community, Competition | Digital reward | -- | ■ | MVPA: Δbetween study groups ↑ Sedentary time: Δbetween study groups ↓ |
| Physical Activity & Sedentary Behavior | ||||||
| 2018 | ||||||
| Buchele Harris H and Chen WY[52] | Both | -- | -- | Video | ◆ ○ |
Post-intervention only: Step count: IG>CG* Light PA: IG>CG Moderate PA: IG>CG* Vigorous PA: IG>CG* Sedentary time: IG<CG* |
| Ezeugwu VE et al.[53] | Static | Phone | -- | One-on-one | N/A | Step count: Δpre/post-intervention ↑ Sedentary time: ΔPP ↓ |
| Li LC et al.[82] | Adaptive | -- | -- | One-on-one or Video | ○W | Step count: Δbetween study groups ↑* MVPA: Δbetween study groups ↑* Sedentary time: Δbetween study groups ↓ |
| Olsen HM et al.[48] | Not specified | Community | -- | Group session, Printed material | N/A | MVPA: Δpre/post-intervention ↓ Light PA: Δpre/post-intervention ↑ Sedentary time: Δpre/post-intervention ↑ |
| Pope ZC et al.[46] | -- | Community | -- | ■ | Step count: Δbetween study groups ↑ MVPA: Δbetween study groups ↑ Light PA: Δbetween study groups ↑ Sedentary time: Δbetween study groups ↑ |
|
| Trinh L et al.[47] | Adaptive | -- | Charitable donation, In-kind | App | N/A | Step count: Δpre/post-intervention ↑* MVPA: Δpre/post-intervention ↑* Light PA: Δpre/post-intervention ↓ Sedentary time: Δpre/post-intervention ↓ |
| 2019 | ||||||
| Lynch BM et al.[54] | Not specified | -- | -- | One-on-one | ○W | Step count: Δbetween study groups ↑ MVPA: Δbetween study groups ↑* Sitting time: Δbetween study groups ↓ |
| Muellmann S et al.[26] | Static | Community | Digital reward | Group session, Printed material | ■ ○W■-only | MVPA: Δbetween study groups ↑ Sedentary time: Δbetween study groups ↓ |
| Sedentary Behavior | ||||||
| 2018 | ||||||
| Guitar NA et al.[83] | Static | -- | -- | One-on-one or Video | N/A | Mean no. of sit-to-stand in an 8hr-workday (goal=16): 12 (post-intervention only) |
| Sleep | ||||||
| 2017 | ||||||
| Kang SG et al.[56] | Adaptive | -- | -- | Video, App | ■ | Sleep efficiency: Δbetween study groups ↑ |
| Physical Activity & Sleep | ||||||
| 2016 | ||||||
| Crowley O et al.[43] | Adaptive | -- | Charitable donation, In-kind, Digital reward | -- | N/A | Step count: Δpre/post-intervention ↓ Sleep duration: Δpre/post-intervention ↑* |
| Melton BF et al.[42] | -- | -- | -- | ■W | Step count: Δbetween study groups ↓* Total PA: Δbetween study groups ↓ Sleep duration: Δbetween study groups ↑ Sleep efficiency: Δbetween study groups ↓ |
|
| Physical Activity, Sedentary Behavior, & Sleep | ||||||
| 2018 | ||||||
| Choi JY et al.[55] | Adaptive | Community, Competition | Cash | One-on-one, Group session, Printed material, Exercise training | ◆ | Step count: Δbetween study groups ↑* Total PA: Δbetween study groups ↑* Sedentary time: Δbetween study groups ↓* Sleep duration: Δbetween study groups ↑ |
Note:
Two articles on the same study, reporting on different outcomes.
Comparison: ◆ - comparison group (CG) received a WAM; ■ - CG received a ‘control intervention’ without a WAM; ○ - CG did not receive anything or received usual care only; ■W – CG received a ‘control intervention’, then after the intervention period was given the WAM; ○W- CG did not receive anything, then after the intervention period was given the WAM; N/A – not applicable (no comparison group)
Findings: Δbetween study groups – difference in the mean change between the IG and CG(s); Δpre/post-intervention – change at post-intervention from baseline; ↑ – increase; ↓ – decrease
– statistically significant (p<.05);
Other commonly used intervention strategies were goal setting (n=54), providing education and/or health coaching (n=41), giving rewards/incentives (n=20), and providing social support. Goals were classified as adaptive (personalized to the participant’s current level and/or adjusted to the participant’s change in level over time) or static (unchanging, e.g. 10,000 steps/day). Only rewards that were meant to encourage the participants to meet PA/SB/sleep goals were considered. Process incentives (e.g., incentives given for completing the assessments, study completion incentives) were not considered. The majority of the interventions used gain-framed incentives, which is providing the reward after specific goals are achieved (n=18). Conversely, the loss-framed approach was only used in two interventions [31, 32]. Social support was provided in the form of encouraging feedback, motivational messages, and identification of barriers and solutions provided by a study team member through email (n=3), text messages (n=8), phone (n=7), and/or in person (n=1). Some interventions also used social support from an in-person (n=8) or online (n=9) community/partner. Another source of social motivation was provided in the form of friendly within-group competition. Interventions that utilized the principles of gamification mainly used the motivational potential of competition to bring about the desired behavioral change [31, 33–36].
Study Outcomes
This scoping review focused only on outcomes related to PA, SB, and sleep (Table 2). Additionally, only objectively-measured findings were abstracted except in a few studies (n=4) that measured their outcomes using only self-report measures [37–40]. Among the studies that used objective instruments, 27 used a different accelerometer (research-grade) from the one used in the intervention. A common strategy among studies that used the same WAM to measure their outcomes was to blind the participants during the baseline measurement (i.e., masking the display or not giving the participants access to their WAM accounts).
Physical Activity Outcomes
Steps.
Out of the 44 studies with interventions that targeted step counts, 31 involved a control group and 13 did not include one. Of the studies with a control group, 25 resulted in a greater increase in steps (14 of which had a statistically significant difference at a p-value of < .05) when compared to the control condition(s). However, 3 studies reported decreased steps at post-intervention—2 in which the pre- to post-intervention decrease in steps was less for the intervention group (p≥.05) [12, 41] and one in which the intervention group decreased steps while the control group increased steps (p<.05) [42]. Among the studies with a control group, 3 compared only the post-intervention step counts with the intervention groups showing greater step counts compared to the control groups (one of which had a statistically difference at a p-value of < .05). Among the studies without a control group, 9 reported increased steps (4 of which had statistically significant difference at a p-value of < .05) and 3 reported a non-significant decrease in steps [43–45]. Lastly, one study that did not have a control group performed a sub-group analysis, wherein the step counts for those who self-selected their group versus those who were assigned to a group were compared [19]. Those who self-selected their group had significantly greater step counts compared to those who were assigned to a group (p< .05) [19].
Moderate-to-vigorous physical activity (MVPA) time.
Twenty-three interventions targeted time spent in MVPA; 16 of these 23 interventions included a control group. Of the studies with a control group, 13 resulted in a greater increase in MVPA time in at least one of the intervention groups compared to the control group (9 of which had statistically significant difference at a p-value of < .05). One study reported a non-statistically significant greater increase in MVPA time in the control group compared to the intervention group [46]. However, 2 studies reported decreased MVPA time at post-intervention—one in which the pre- to post-intervention decrease in MVPA time was less for the intervention group (p≥.05)[28] and one in which the decrease was greater for the intervention group (p≥.05)[12]. Among the 7 studies that did not have a control group, 5 reported an increase in MVPA time at post-intervention (one of which had statistically significant increase at a p-value of < .05[47]) and 2 showed a non-significant decrease in MVPA time [45, 48].
Total PA time.
Eleven interventions targeted time spent in PA; 8 of these 11 interventions included a control group. Of the 8 studies with a control group, 7 resulted in a greater increase in total PA time in the intervention group compared to the control group (5 of which had statistically significant difference at a p-value of < .05). On the other hand, one study reported a non-significant decrease in total PA time for the intervention group relative to control [42]. Among the 3 studies without a control group, one reported a significant increase in total PA time [23], another had a non-significant increase [49], and one reported a non-significant decrease in total PA time [50].
Sedentary Behavior Outcomes
Sedentary time.
Twelve interventions targeted sedentary time; 8 of these interventions included a control group. Of the 8 studies with a control group, 5 resulted in a greater decrease in sedentary time in the intervention group compared to the control group (2 of which had statistically significant difference at a p-value of < .05). However, 2 studies reported a non-significant increase in sedentary time [46, 51]. One study that examined only post-intervention findings reported that intervention group spent significantly less time being sedentary compared to the control group [52]. Of the 4 studies without a control group, 3 reported a non-significant decrease in sedentary time [47, 49, 53] and one reported a non-significant increase in sedentary time [48].
Sitting time.
Only 2 studies explored sitting time. One reported a significant decrease in sitting time in the intervention group compared to the control group [39]. The other intervention also resulted in a greater decrease in sitting time for the intervention group; however, the difference was not significant [54].
Sleep Outcomes
Sleep duration.
Three interventions targeted sleep duration; 2 of these interventions included a control group. Two interventions resulted in greater, albeit non-significant, increases in sleep duration in the intervention group compared to the control group [42, 55]. The other study reported a significant increase in sleep duration post-intervention compared to baseline [43].
Sleep efficiency.
Sleep efficiency (defined as the ratio of sleep duration to time spent in bed) was targeted in 2 studies. One study reported a non-significant increase in sleep efficiency in the intervention group compared to the control group [56]. The second study reported a non-significant decrease in sleep efficiency [42].
Device Adherence
Out of the 61 studies, 28 provided information on device adherence. Device adherence was reported as the percentage of participants who wore their WAM, the proportion of days the participants wore their WAM, or a combination of the two. Similarly, valid “wear time” was also operationalized in numerous ways (e.g., 500 steps/day for 4 days, 10 hours/day for 5 days, 8 hours/day for 3 days), making synthesizing the findings a challenge. A descriptive summary of device adherence for the 28 studies is outlined in Online Resource 2.
Discussion
The overwhelming majority of the WAM-based interventions focused on increasing PA, which was commonly operationalized using step count, MVPA time, and/or total PA time. Interventions designed to decrease SB were much less common, and even less frequent were interventions designed to improve sleep. Of the 44 studies that targeted step count, 18 resulted in statistically significant increases in steps post-intervention. Of the 23 studies that targeted MVPA time, 10 saw statistically significant increases in MVPA time post-intervention. Similarly, 6 of the 11 studies that targeted total PA time resulted in significant increases in total PA time. Among the studies that targeted sedentary behavior, 2 of 12 studies resulted in significant decreases in sedentary time and 1 of 2 studies resulted in a significant decrease in sitting time. Among the studies that focused on sleep, 1 of 3 resulted in a significant increase in sleep duration; however, none of the studies significantly improved sleep efficiency. It should be noted that most of the studies examined the feasibility of WAM-based interventions and/or explored its preliminary efficacy; hence, the efficacy of the WAM-based interventions could not be conclusively determined. Nonetheless, WAM-based interventions exhibited considerable potential in increasing PA and decreasing SB across a diverse range of populations. This is especially true for multicomponent interventions that incorporated behavior change strategies, not only to encourage adoption of the desired behavior, but also to increase adherence to the intervention.
A quantitative synthesis of device adherence could not be performed due to the heterogenous metrics used to determine device adherence. However, what was apparent is that a number of studies had issues with device adherence. Unfortunately, participants discontinued use of the WAM quite often, typically after a few months when the novelty of the WAM dissipated [57]. Novelty effect—defined as the individual’s initial response to the technology rather than the pattern of use over time when habituation settles in [57]—was observed in several of the studies. After observing a significant initial increase in physical activity, a steady decline was noted in several studies. To minimize the potential bias from the novelty effect, one study included a run-in week to allow the participants to become accustomed to the WAM, and then excluded data collected during that week [31]. While this brief period provided a time to become comfortable with the device, it is not clear that one week is sufficient to remove the novelty effect. Further study needs to be done to examine what strategies can be implemented to provide more sustained use and engagement of these consumer friendly WAM. A recent editorial reported on this dilemma and suggested a novel approach to address this recurring issue [58].
Another common issue faced in the WAM-based interventions was the rapid turnover of the direct-to-consumer WAM. Since most of the WAM included in this review have already been discontinued, it is apparent that traditional research designs cannot keep up with the typical WAM lifecycle. This calls for more innovative designs and shorter study cycles to establish any efficacy of the WAM [59, 60].
While this scoping review was conducted following the PRISMA-ScR guidelines, it is not without limitations. First, the electronic search was limited to three databases, which could have introduced selection bias risk. To minimize this risk, a manual search was conducted to complement the electronic search and the electronic search strategy was formulated with the assistance of an experienced reference librarian. Additionally, critical appraisal of the risk of bias in the included studies was not undertaken. While optional for scoping reviews, formal assessment of bias increases the rigor of scoping reviews. However, there are also strengths to our review. First, we included studies that targeted very diverse population groups across the age span and range of disease or health conditions, and we reviewed studies that tested an array of wearable devices.
In conclusion, the efficacy of WAM-based interventions still needs to be established using rigorous study designs and adequate sample sizes. Further, WAM-based interventions targeting sleep are limited compared to interventions designed to improve PA and/or address SB. Nonetheless, based on the included studies, WAM-based interventions are feasible and acceptable for various populations, and show promising potential in increasing PA and reducing SB.
Supplementary Material
Acknowledgement:
We would like to thank our reference librarian, Rebecca Raszewksi, MS, AHIP for her assistance in developing the electronic search strategy.
Footnotes
Conflict of Interest: Dr. Kline reports grants from National Institutes of Health outside of the submitted work. The other authors declare no conflicts of interest.
Human and Animal Rights: This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Maan Isabella Cajita, University of Illinois at Chicago, College of Nursing, 845 S. Damen Ave., Chicago, IL, USA.
Christopher E. Kline, University of Pittsburgh, Department of Health and Physical Activity, Pittsburgh, PA, USA.
Lora E. Burke, University of Pittsburgh, School of Nursing, Pittsburgh, PA, USA.
Evelyn G. Bigini, University of Pittsburgh, School of Nursing, Pittsburgh, PA, USA
Christopher C. Imes, University of Pittsburgh, School of Nursing, Pittsburgh, PA, USA.
References
- 1.Wright SP, et al. , How consumer physical activity monitors could transform human physiology research. Am J Physiol Regul Integr Comp Physiol, 2017. 312(3): p. R358–R367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Data Corportation, Wrist-worn wearables maintain a strong growth trajectory in Q2 2019, according to IDC. 2019, Press release from IDC on September 12, 2019..
- 3.Omura JD, et al. , National physical activity surveillance: Users of wearable activity monitors as a potential data source. Prev Med Rep, 2017. 5: p. 124–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henriksen A, et al. , Using fitness trackers and smartwatches to measure physical activity in research: analysis of consumer wrist-worn wearables. J Med Internet Res, 2018. 20(3): p. e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Straiton N, et al. , The validity and reliability of consumer-grade activity trackers in older, community-dwelling adults: A systematic review. Maturitas, 2018. 112: p. 85–93. [DOI] [PubMed] [Google Scholar]
- 6.Evenson KR, Goto MM, and Furberg RD, Systematic review of the validity and reliability of consumer-wearable activity trackers. Int J Behav Nutr Phys Act, 2015. 12: p. 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang SG, et al. , Validity of a commercial wearable sleep tracker in adult insomnia disorder patients and good sleepers. J Psychosom Res, 2017. 97: p. 38–44. [DOI] [PubMed] [Google Scholar]
- 8.de Zambotti M, et al. , Wearable Sleep Technology in Clinical and Research Settings. Med Sci Sports Exerc, 2019. 51(7): p. 1538–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Bruin E, et al. , Wearable systems for monitoring mobility-related activities in older people: a systematic review. Clinical Rehabilitation, 2008. 22: p. 878–895. [DOI] [PubMed] [Google Scholar]
- 10.Ridgers ND, McNarry MA, and Mackintosh KA, Feasibility and effectiveness of using wearable activity trackers in youth: a systematic review. JMIR Mhealth Uhealth, 2016. 4(4): p. e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tricco AC, et al. , PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med, 2018. 169(7): p. 467–473. [DOI] [PubMed] [Google Scholar]
- 12.Evans EW, et al. , Using novel technology within a school-based setting to increase physical activity: a pilot study in school-age children from a low-income, urban community. BioMed Research International, 2017. 2017: p. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cadmus-Bertram LA, et al. , Randomized trial of a Fitbit-based physical activity intervention for women. American Journal of Preventive Medicine, 2015. 49(3): p. 414–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cadmus-Bertram L, et al. , Use of the Fitbit to measure adherence to a physical activity intervention among overweight or obese, postmenopausal women: self-monitoring trajectory during 16 weeks. Journal of Medical Internet Research, 2015. 17(11): p. 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sloan RA, et al. , The influence of a consumer-wearable activity tracker on sedentary time and prolonged sedentary bouts: secondary analysis of a randomized controlled trial. BMC Res Notes, 2018. 11(1): p. 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finkelstein EA, et al. , Effectiveness of activity trackers with and without incentives to increase physical activity (TRIPPA): a randomised controlled trial. The Lancet Diabetes and Endocrinology, 2016. 4(12): p. 983–995.• A large randomized-controlled trial (RCT) that compared the impact of WAM with or without modest incentives on physical activity (PA) in generally healthy adults. Cash incentive with WAM was most effective in increasing PA; however, this effect was not sustained. This study exemplifies the challenge of sustained device engagement that plagued several WAM-based interventions.
- 17.Phillips CB, Hurley JC, Angadi SS, et al. Delay discount rate moderates a physical activity intervention testing immediate rewards. Behavioral Medicine. 2020;46(2):142–152. doi: 10.1080/08964289.2019.1570071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams MA, et al. , Adaptive goal setting and financial incentives: a 2 × 2 factorial randomized controlled trial to increase adults’ physical activity. BMC Public Health, 2017. 17(1): p. 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meints SM, et al. , Race differences in physical activity uptake within a workplace wellness program: a comparison of Black and White employees. American Journal of Health Promotion, 2019. 33(6): p. 886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Losina E, et al. , Implementation of a workplace intervention using financial rewards to promote adherence to physical activity guidelines: a feasibility study. BMC Public Health, 2017. 17: p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barwais FA, Cuddihy TF, and Tomson LM, Physical activity, sedentary behavior and total wellness changes among sedentary adults: a 4-week randomized controlled trial. Health Qual Life Outcomes, 2013. 11: p. 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barwais FA and Cuddihy TF, Empowering sedentary adults to reduce sedentary behavior and increase physical activity levels and energy expenditure: a pilot study. Int J Environ Res Public Health, 2015. 12(1): p. 414–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abrantes AM, et al. , Developing a Fitbit-supported lifestyle physical activity intervention for depressed alcohol dependent women. Journal of Substance Abuse Treatment, 2017. 80: p. 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cadmus-Bertram L, et al. , Building a physical activity intervention into clinical care for breast and colorectal cancer survivors in Wisconsin: a randomized controlled pilot trial. Journal of Cancer Survivorship, 2019. 13(4): p. 593–602.• A pilot study that tested the impact of a multicomponent WAM-based intervention on PA in cancer survivors. The intervention led to significant increase in PA. This study exemplifies the feasibility of WAM-based PA interventions for individuals with health conditions.
- 25.Poirier J, et al. , Effectiveness of an activity tracker- and Internet-based adaptive walking program for adults: a randomized controlled trial. Journal of medical Internet research, 2016. 18(2): p. e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muellmann S, et al. , Effects of two web-based interventions promoting physical activity among older adults compared to a delayed intervention control group in Northwestern Germany: results of the PROMOTE community-based intervention trial. Preventive Medicine Reports, 2019. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi J, et al. , mHealth physical activity intervention: a randomized pilot study in physically inactive pregnant women. Maternal and child health journal, 2016. 20(5): p. 1091–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amorim AB, Pappas E, Simic M, et al. Integrating mobile-health, health coaching, and physical activity to reduce the burden of chronic low back pain trial (IMPACT): a pilot randomised controlled trial. BMC Musculoskeletal Disorders. 2019;20(1):71. doi: 10.1186/s12891-019-2454-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deka P, et al. , MOVE-HF: an internet-based pilot study to improve adherence to exercise in patients with heart failure. European Journal of Cardiovascular Nursing, 2019. 18(2): p. 122–131. [DOI] [PubMed] [Google Scholar]
- 30.Adams MA, et al. , Adaptive goal setting and financial incentives: a 2 × 2 factorial randomized controlled trial to increase adults’ physical activity. BMC Public Health, 2017. 17: p. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel MS, et al. , Effect of a game-based intervention designed to enhance social incentives to increase physical activity among families: the BE FIT randomized clinical trial. JAMA Intern Med, 2017. 177(11): p. 1586–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chokshi NP, et al. , Loss-framed financial incentives and personalized goal-setting to increase physical activity among ischemic heart disease patients using wearable devices: the ACTIVE REWARD randomized trial. Journal of the American Heart Association, 2018. 7(12): p. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garde A, et al. , Assessment of a mobile game (“MobileKids Monster Manor”) to promote physical activity among children. Games Health J, 2015. 4(2): p. 149–58. [DOI] [PubMed] [Google Scholar]
- 34.Garde A, et al. , Evaluation of a novel mobile exergame in a school-based environment. Cyberpsychol Behav Soc Netw, 2016. 19(3): p. 186–92.• A novel study that tested the impact of exergaming, which combines PA with electronic games, in children. Participants earned “game time” by engaging in PA. The intervention led to significant increase in PA. This study exemplifies the feasibility of WAM-based interventions in children.
- 35.Gremaud AL, et al. , Gamifying accelerometer use increases physical activity levels of sedentary office workers. Journal of the American Heart Association, 2018. 7(13): p. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung AE, et al. , Tweeting to health: a novel mHealth intervention using Fitbits and Twitter to foster healthy lifestyles. Clinical Pediatrics, 2017. 56(1): p. 26–32. [DOI] [PubMed] [Google Scholar]
- 37.Shin DW, et al. , Enhancing physical activity and reducing obesity through smartcare and financial incentives: A pilot randomized trial. Obesity (19307381), 2017. 25(2): p. 302–310. [DOI] [PubMed] [Google Scholar]
- 38.Barwais FA, Cuddihy TF, and Tomson LM, Physical activity, sedentary behavior and total wellness changes among sedentary adults: a 4-week randomized controlled trial. Health and Quality of Life Outcomes, 2013. 11(183): p. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vandelanotte C, et al. , The effectiveness of a web-based computer-tailored physical activity intervention using Fitbit activity trackers: randomized trial. Journal of Medical Internet Research, 2018. 20(12): p. 30–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janevic MR, Shute V, Murphy SL, Piette JD. Acceptability and effects of commercially available activity trackers for chronic pain management among older African American adults. Pain Med. 2020;21(2):e68–e78. doi: 10.1093/pm/pnz215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polgreen LA, et al. , The effect of automated text messaging and goal setting on pedometer adherence and physical activity in patients with diabetes: A randomized controlled trial. PLoS One, 2018. 13(5): p. e0195797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melton BF, et al. , Wearable devices to improve physical activity and sleep. Journal of Black Studies, 2016. 47(6): p. 610–625. [Google Scholar]
- 43.Crowley O, Pugliese L, and Kachnowski S, The impact of wearable device enabled health initiative on physical activity and sleep. Cureus, 2016. 8(10): p. e825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hooke MC, et al. , Use of a fitness tracker to promote physical activity in children with acute lymphoblastic leukemia. Pediatric Blood & Cancer, 2016. 63(4): p. 684–689. [DOI] [PubMed] [Google Scholar]
- 45.Gell NM, et al. , Efficacy, feasibility, and acceptability of a novel technology-based intervention to support physical activity in cancer survivors. Supportive Care in Cancer, 2017. 25(4): p. 1291–1300. [DOI] [PubMed] [Google Scholar]
- 46.Pope ZC, Zeng N, Zhang R, Lee HY, Gao Z. Effectiveness of combined smartwatch and social media intervention on breast cancer survivor health outcomes: A 10-week pilot randomized trial. Journal of Clinical Medicine. 2018;7(6):140. doi: 10.3390/jcm7060140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trinh L, et al. , RiseTx: testing the feasibility of a web application for reducing sedentary behavior among prostate cancer survivors receiving androgen deprivation therapy. International Journal of Behavioral Nutrition & Physical Activity, 2018. 15(1): p. N.PAG-N.PAG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olsen HM, et al. , A brief self-directed intervention to reduce office employees’ sedentary behavior in a flexible workplace. Journal of Occupational & Environmental Medicine, 2018. 60(10): p. 954–959. [DOI] [PubMed] [Google Scholar]
- 49.Gilson ND, et al. , The impact of an m-Health financial incentives program on the physical activity and diet of Australian truck drivers. BMC Public Health, 2017. 17: p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hacker ED, et al. , Steps to enhance early recovery after hematopoietic stem cell transplantation: lessons learned from a physical activity feasibility study. Clinical nurse specialist CNS, 2018. 32(3): p. 152–162. [DOI] [PubMed] [Google Scholar]
- 51.Sloan RA, et al. , The influence of a consumer-wearable activity tracker on sedentary time and prolonged sedentary bouts: secondary analysis of a randomized controlled trial. BMC research notes, 2018. 11(1): p. 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buchele H, Chen W. Technology-enhanced classroom activity breaks impacting children’s physical activity and fitness. Journal of Clinical Medicine. 2018;7(7):165. doi: 10.3390/jcm7070165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ezeugwu VE and Manns PJ, The feasibility and longitudinal effects of a home-based sedentary behavior change intervention after stroke. Arch Phys Med Rehabil, 2018. 99(12): p. 2540–2547. [DOI] [PubMed] [Google Scholar]
- 54.Lynch BM, et al. , Maintenance of physical activity and sedentary behavior change, and physical activity and sedentary behavior change after an abridged intervention: Secondary outcomes from the ACTIVATE Trial. Cancer (0008543X), 2019. 125(16): p. 2856–2860. [DOI] [PubMed] [Google Scholar]
- 55.Choi JY, Chang AK, and Choi EJ, Effects of a physical activity and sedentary behavior program on activity levels, stress, body size, and sleep in sedentary Korean college students. Holist Nurs Pract, 2018. 32(6): p. 287–295.• A quasi-experimental study that examined the effect of a WAM-based intervention on PA, sedentary behavior, and sleep. The intervention led to significant increase in PA, a significant decrease in sedentary time, and a non-significant increase in sleep duration.
- 56.Kang SG, et al. , Cognitive behavioral therapy using a mobile application synchronizable with wearable devices for insomnia treatment: a pilot study. J Clin Sleep Med, 2017. 13(4): p. 633–640.• A pilot study that tested the efficacy of a technology-based sleep intervention and the only one that focused on individuals with insomnia. The WAM was used to objectively track sleep which was then used to inform the sleep prescription by the therapist.
- 57.Shin G, et al. , Beyond novelty effect: a mixed-methods exploration into the motivation for long-term activity tracker use. JAMIA Open, 2019. 2(1): p. 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cohen J, Torous J. The potential of object-relations theory for improving engagement with health apps. JAMA. 2019;322(22):2169–2170. doi: 10.1001/jama.2019.17141 [DOI] [PubMed] [Google Scholar]
- 59.Pellegrini CA, Steglitz J, and Hoffman SA, e-Health intervention development: a synopsis and comment on “what design features are used in effective e-Health interventions? a review using techniques from critical interpretive synthesis”. Transl Behav Med, 2014. 4(4): p. 342–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nahum-Shani I, et al. , Experimental design and primary data analysis methods for comparing adaptive interventions. Psychol Methods, 2012. 17(4): p. 457–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hayes LB, Van Camp CM. Increasing physical activity of children during school recess. Journal of applied behavior analysis. 2015;48(3):690–695. [DOI] [PubMed] [Google Scholar]
- 62.Martin SS, Feldman DI, Blumenthal RS, et al. mActive: A randomized clinical trial of an automated mHealth intervention for physical activity promotion. Journal of the American Heart Association. 2015;4(11):e002239. doi: 10.1161/JAHA.115.002239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Le A, Mitchell HR, Zheng DJ, et al. A home-based physical activity intervention using activity trackers in survivors of childhood cancer: A pilot study. Pediatric Blood and Cancer. 2016;64(2):387–394. [DOI] [PubMed] [Google Scholar]
- 64.McMahon SK, Lewis B, Oakes JM, Wyman JF, Guan W, Rothman AJ. Assessing the effects of interpersonal and intrapersonal behavior change strategies on physical activity in older adults: a factorial experiment. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2017;51(3):376–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rote AE. Physical activity intervention using Fitbits in an introductory college health course. Health Education Journal. 2017;76(3):337–348. [Google Scholar]
- 66.Yeung J, Mazloomdoost D, Crisp CC, Kleeman S, Pauls RN. Impact of electronic feedback and peer comparisons on residents’ physical activity level. Journal of graduate medical education. 2017;9(4):527–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang X, McClean D, Ko E, Morgan MA, Schmitz K. Exercise among women with ovarian cancer: a feasibility and pre-/post-test exploratory pilot study. Oncology nursing forum. 2017;44(3):366–374. [DOI] [PubMed] [Google Scholar]
- 68.Bade BC, Hyer JM, Bevill BT, et al. A patient-centered activity regimen improves participation in physical activity interventions in advanced-stage lung cancer. Integrative Cancer Therapies. 2018;17(3):921–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.DiFrancisco-Donoghue J, Jung MK, Stangle A, et al. Utilizing wearable technology to increase physical activity in future physicians: A randomized trial. Preventive Medicine Reports. 2018;12:122–127. doi: 10.1016/j.pmedr.2018.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Duscha BD, Piner LW, Patel MP, et al. Effects of a 12-week mHealth program on peak VO2 and physical activity patterns after completing cardiac rehabilitation: A randomized controlled trial. American Heart Journal. 2018;199:105–114. [DOI] [PubMed] [Google Scholar]
- 71.Heale LD, Dover S, Goh YI, Maksymiuk VA, Wells GD, Feldman BM. A wearable activity tracker intervention for promoting physical activity in adolescents with juvenile idiopathic arthritis: A pilot study. Pediatric Rheumatology Online J. 2018;16(1):66. doi: 10.1186/s12969-018-0282-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kooiman TJM, de Groot M, Hoogenberg K, Krijnen WP, van der Schans CP, Kooy A. Self-tracking of physical activity in people with type 2 diabetes: a randomized controlled trial. CIN: Computers, Informatics, Nursing. 2018;36(7):340–349. [DOI] [PubMed] [Google Scholar]
- 73.Liau AK, Neihart M, Teo CT, Goh LS, Chew P. A quasi-experimental study of a Fitbit-based self-regulation intervention to improve physical activity, well-being, and mental health. Cyberpsychology, behavior and social networking. 2018;21(11):727–734. [DOI] [PubMed] [Google Scholar]
- 74.McDermott MM, Spring B, Berger JS, et al. Effect of a home-based exercise intervention of wearable technology and telephone coaching on walking performance in peripheral artery disease: the HONOR randomized clinical trial. JAMA. 2018;319(16):1665–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nyrop KA, Deal AM, Choi SK, et al. Measuring and understanding adherence in a home-based exercise intervention during chemotherapy for early breast cancer. Breast Cancer Research and Treatment. 2018;168(1):43–55. [DOI] [PubMed] [Google Scholar]
- 76.Ovans JA, Hooke MC, Bendel AE, Tanner LR. Physical therapist coaching to improve physical activity in children with brain tumors: a pilot study. Pediatric Physical Therapy. 2018;30(4):310–317. [DOI] [PubMed] [Google Scholar]
- 77.Yoon S, Schwartz JE, Burg MM, et al. Using behavioral analytics to increase exercise: a randomized n-of-1 study. American Journal of Preventive Medicine. 2018;54(4):559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cheung NW, Blumenthal C, Smith BJ, et al. A pilot randomised controlled trial of a text messaging intervention with customisation using linked data from wireless wearable activity monitors to improve risk factors following gestational diabetes. Nutrients. 2019;11(3):590. doi: 10.3390/nu11030590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Christiansen MB, Thoma LM, Master H, et al. The feasibility and preliminary outcomes of a physical therapist-administered physical activity intervention after total knee replacement. Arthritis care & research. 2020;72(5):661–668. doi: 10.1002/acr.23882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Van Blarigan EL, Chan H, Van Loon K, et al. Self-monitoring and reminder text messages to increase physical activity in colorectal cancer survivors (Smart Pace): a pilot randomized controlled trial. BMC Cancer. 2019;19(1):218–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mendoza JA, Baker KS, Moreno MA, et al. A Fitbit and Facebook mHealth intervention for promoting physical activity among adolescent and young adult childhood cancer survivors: A pilot study. Pediatric Blood and Cancer. 2017;64(12):e26660. doi: 10.1002/pbc.26660 [DOI] [PubMed] [Google Scholar]
- 82.Li LC, Sayre EC, Xie H, et al. Efficacy of a community-based technology-enabled physical activity counseling program for people with knee osteoarthritis: proof-of-concept study. Journal of Medical Internet Research. 2018;20(4):e159. doi: 10.2196/jmir.8514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guitar NA, MacDougall A, Connelly DM, Knight E. Fitbit activity trackers interrupt workplace sedentary behavior: a new application. Workplace Health & Safety. 2018;66(5):218–222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.