Figure 2. Nuclear import of histones.
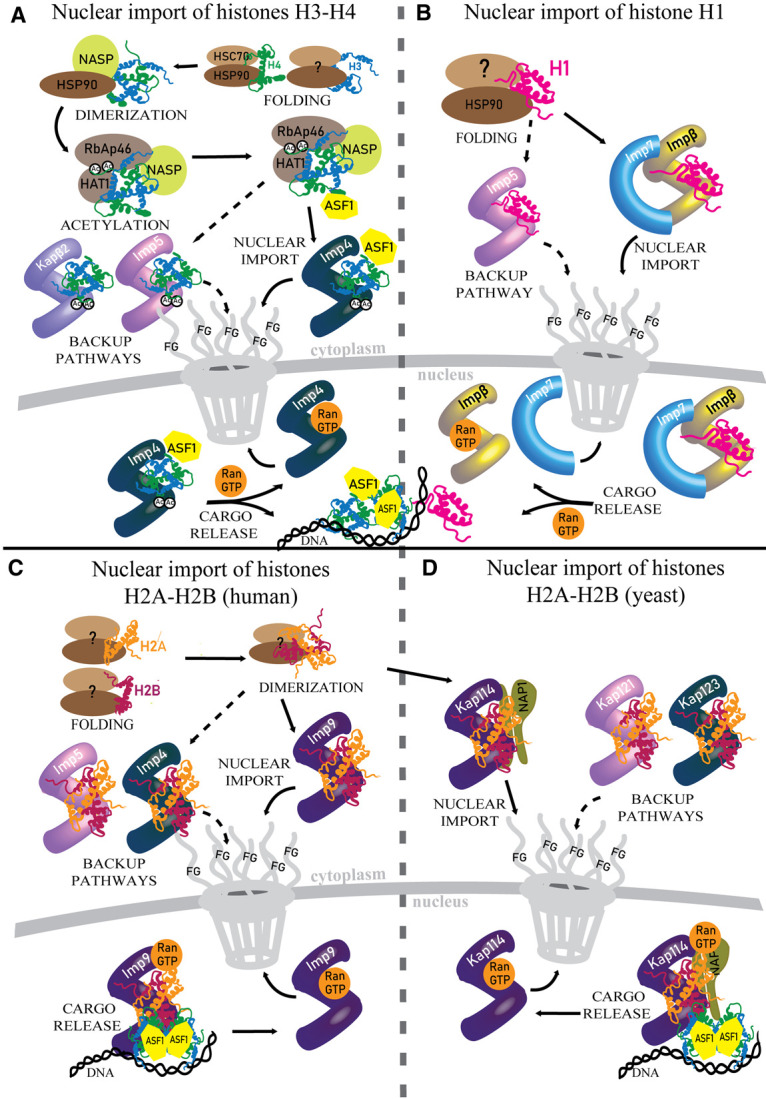
(A) Schematic showing nuclear import of H3–H4: after translation, H3 (blue) and H4 (green) are folded by HSC70 and HSP90 and then dimerized by NASP and HSP90. NASP then recruits RBAP46/HAT1 to acetylate K5 and K12 residues in the H4 tail. The H3–H4 heterodimer then binds ASF1 and Imp4 for nuclear import, and also bind secondary nuclear importers Imp5 and Kapβ. Imp4•H3–H4•Asf1 is shown translocating through the NPC into the nucleus where RanGTP binds Imp4 to release H3–H4•Asf1 for nucleosome assembly. (B) Nuclear import of H1 (pink). Little is known about how H1 is processed from synthesis to nuclear import, but it is well established that the Impβ/Imp7 heterodimer imports H1. After translocation through the NPC, FG repeats of nucleoporins bind Imp7 to release Impβ, and RanGTP binds Impβ to release H1. (C) In human cells, H2A–H2B (yellow-red) are imported by Imp9, with Imp4 and Imp5 as secondary importers. In the nucleus, RanGTP binds to Imp9•H2A–H2B without releasing the histones. RanGTP•Imp9•H2A–H2B binds DNA and effectively deposits H2A–H2B into the assembling nucleosome. (D) Kap114 (yeast homolog of Imp9) imports H2A–H2B and NAP1. Kap114•H2A–H2B•Nap1 is insensitive to RanGTP, with H2A–H2B and NAP1 released only in the presence of DNA.
