Abstract
Background
Infection is the most common non-cardiovascular cause of re-hospitalizations for heart failure patients. We therefore investigated the predictors of infection-related re-hospitalization (IRRH) in heart failure patients and its impact on long-term survival.
Methods and Results
We prospectively recruited 622 patients after the index hospitalization for decompensated heart fail with primary endpoints of IRRH and all-cause mortality. During follow-up of 3.9 ± 2.7 years, IRRHs occurred in 104 (16.7%) patients. Of the 104 patients who experienced IRRHs, the time from the index hospitalization to IRRH was 1.0 (interquartile range: 0.4–2.6) years. Independent predictors of IRRH were age (hazard ratio: 1.02, 95% confidence interval: 1.01–1.04), diabetes mellitus (2.12, 1.42–3.17), not taking angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers (1.67, 1.01–2.78), needing maintenance therapy with a loop diuretic (2.10, 1.36–3.26), hemoglobin levels (0.87, 0.79–0.96), and estimated glomerular filtration rates (eGFRs) (0.99, 0.98–0.99). IRRH independently predicted all-cause mortality (1.99, 1.32–2.98) after adjusting for age, body mass index, New York Heart Association functional class, chronic obstructive pulmonary disease, brain natriuretic peptide, hemoglobin, and eGFR. The increased risk of death associated with IRRHs was predominantly for lower respiratory tract infections (3.71, 2.28–6.04), urogenital tract infections (2.83, 1.32–6.10), and sepsis (3.26, 1.20–8.85).
Conclusion
IRRHs in patients discharged for acute decompensated heart fail independently predicted worse long-term survival. We further identified independent predictors of IRRHs. These findings warrant future studies for tackling IRRH.
Keywords: heart failure, hospitalization, infection, mortality, risk factor
Introduction
Heart failure is a global healthcare issue affecting an estimated 26 million people worldwide.1 Even though there have been significant advances in understanding the pathophysiology and therapeutic strategies for heart failure, the mortality rate remains high.2 Heart failure rehospitalizations after discharge are common and are significantly associated with subsequent mortality.3,4 More than 60% of all rehospitalizations following discharge for heart failure have noncardiovascular causes; of these, infection is the most common.5 However, there have been no prospective studies designed to untangle the risk factors for infection-related rehospitalization (IRRH) after decompensated heart failure or studies delineating the association between IRRH and long-term survival. A better understanding of risk factors for IRRH in heart failure patients can help reduce the rehospitalization rate and potentially improve long-term survival. This study aimed to identify predictors of IRRH in patients discharged for acute decompensated heart failure and its impact on long-term survival based on the cohort of patients enrolled at our heart failure center over the past 10 years.
Methods
Patients
The current study used a prospective observational design with long-term follow-up. We consecutively recruited and followed patients up after their index hospitalization for decompensated heart failure at the Heart Failure Center of Chang Gung Memorial Hospital in Keelung, Taiwan from 1 October 2008 to 30 June 2018. The detailed information of the multidisciplinary disease management program and longitudinal follow-up design for heart failure patients have been described in our previous study.6 As previously reported, the enrollment criteria were: first, patients with typical symptoms and signs of heart failure who were hospitalized due to acute cardiogenic pulmonary congestion determined by chest radiographs (grade ≥ I) according to the classification by Battler et al.,7 second, patients with abnormal cardiac structure documented by echocardiograms, and third, patients from ages 20 to 85. The exclusion criteria were: first, patients with a disorder other than heart failure that might compromise their survival within less than 6 months, second, patients who were bedridden for at least 3 months, third, patients who had undergone dialysis in the previous 2 weeks, and fourth, patients who were pregnant.
Echocardiography
All patients received two-dimensional echocardiography assessments (GE Vivid E9) during the index hospitalization for decompensated heart failure according to the guidelines suggested by the American Society of Echocardiography.8 Echocardiographic images were obtained with patients in the left lateral decubitus position. The left ventricular ejection fraction was calculated using the Simpson method. In patients having jets of tricuspid regurgitation clearly detected by continuous-wave Doppler ultrasound, we measured the peak tricuspid regurgitation velocity. Given the inaccuracy of right atrium (RA) pressure estimation, we measured the peak tricuspid regurgitation velocity but not the estimated systolic pulmonary artery pressure calculated by tricuspid regurgitation velocity and RA pressure.9 Patients with peak tricuspid regurgitation velocity at least 2.9 m/s were categorized as having a high probability of pulmonary hypertension.9
Heart failure with reduced, mid-range and, preserved ejection fraction
Patients were categorized as having heart failure with reduced ejection fraction (HFrEF), heart failure with mid-range ejection fraction, and heart failure with preserved ejection fraction if the left ventricle ejection fraction was less than 40%, 40–49%, and at least 50%, respectively, according to the 2016 European Society of Cardiology guidelines for the diagnosis and treatment of acute and chronic heart failure.10
Pharmacological treatment for heart failure patients
Angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) and beta blockers were initiated in all patients during the index hospitalization as appropriate, unless contraindicated or not tolerated. We tapered, then discontinued loop diuretics for patients who could maintain euvolemia status well after receiving instructions about salt/fluid restriction during the index hospitalization to avoid the potential side effects of loop diuretics. If loop diuretics were discontinued, the patient was instructed to take loop diuretics again only if there were symptoms/signs of congestion or a significant increase in daily body weight.6,10,11 Maintenance therapy with a loop diuretic (MTLD) was defined as taking a loop diuretic daily among patients whose fluid status cannot be controlled by salt and fluid restriction alone.
We assessed potential clinical predictors of IRRH at the index hospitalization for decompensated heart failure. The brain natriuretic peptide was checked before discharge of the index hospitalization for decompensated heart failure. Informed consent was obtained from all patients in the study. The study was designed and carried out in accordance with the principles of the Declaration of Helsinki and with approval from the Ethics Review Board of Chang Gung Memorial Hospital.
Patient outcomes
Follow-up data were obtained prospectively every month from hospital records, telephone interviews with patients, and communication with physicians in charge. The primary endpoints included IRRH and all-cause mortality. Patients were followed until they died, the study ended, or they were lost to follow-up. In each case, whether or not the subsequent hospitalizations were due to infection was determined by two cardiologists after communicating with the physicians in charge. IRRHs were categorized into several types: lower respiratory tract infection, soft tissue or muscular skeletal system infection, urogenital tract infections, gastrointestinal tract infection, and sepsis. A similar approach to categorizing infections among hospitalized heart failure patients had been used previously.5 For patients who experienced IRRHs several times, only the first event was included in univariate and multivariable analysis.
Statistical analysis
Results are expressed as the mean ± SD for normally distributed variables, medians (lower quartile; upper quartile) for variables with skewed distribution, and number (percentage) for categorical variables. We used the Cox regression with backward selection analysis to assess the effects of different variables on the first IRRH. Variables with P value less than 0.05 in the univariate analysis were selected for the multivariable analysis. Hazard ratios and 95% confidence intervals (CIs) were also calculated. Since IRRH was a time-varying exposure in this cohort, we assessed the impact of IRRH on long-term survival by using time-updated Cox proportional hazard models.12 We performed Kaplan–Meier analyses and determined statistical significance using the log-rank test. The receiver operating characteristic curve and Youden's index were used to identify the cutoff values of hemoglobin (Hb). A P value of less than 0.05 was considered significant. Statistical analyses were performed using SPSS software, version 17.0. (SPSS Inc. Released 2008. SPSS Statistics for Windows, Version 17.0. Chicago: SPSS Inc.).
Results
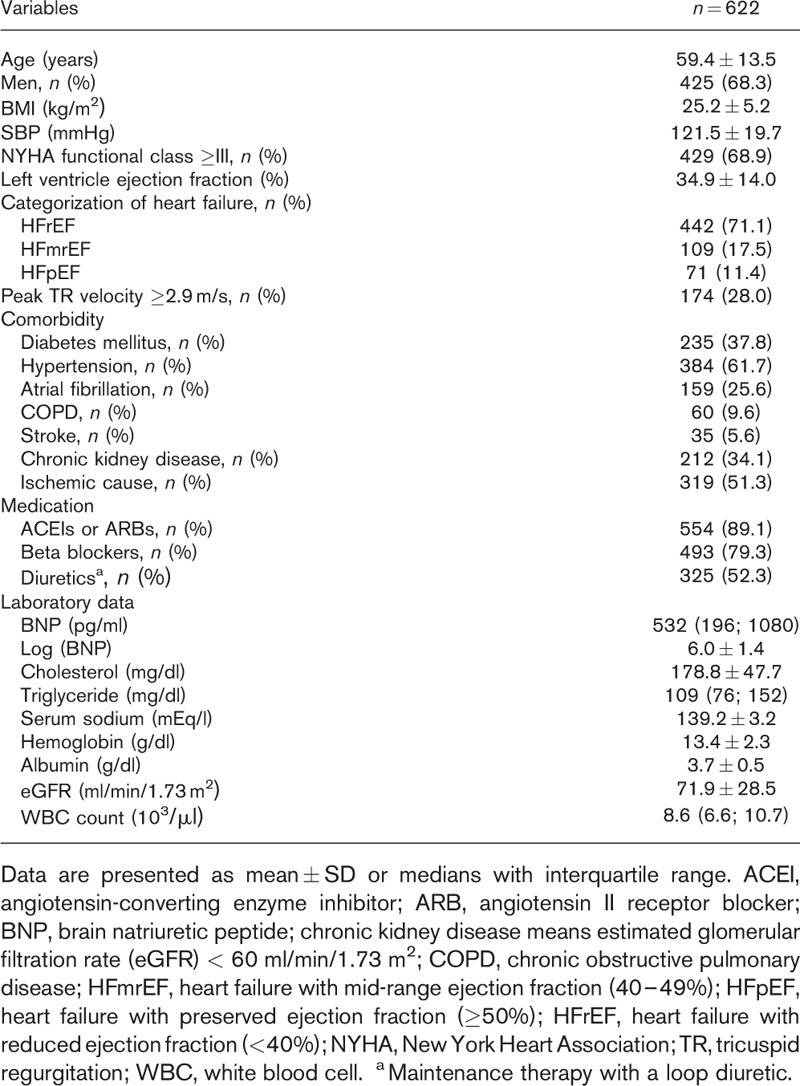
A total of 622 patients were recruited for this study. Their baseline characteristics are shown in Table 1. The patients’ mean age was 59.4 years. Most patients were men, in New York Heart Association functional classes III–IV, and had HFrEF. These patients had multiple comorbidities. More than half had hypertension or ischemic heart disease, more than one-third had chronic kidney disease or diabetes mellitus, and more than one-quarter had atrial fibrillation. At discharge, around 80% of them took ACEIs (or ARBs), beta-blockers and more than half needed MTLD. There were 174 (28%) patients with peak tricuspid regurgitation velocity at least 2.9 m/s. The median of the brain natriuretic peptide levels was 532 pg/ml.
Table 1.
Baseline clinical characteristics
The follow-up duration was 3.9 ± 2.7 years, with the longest follow-up of 9.7 years. For each year from 1 to 9 after patients enrolled, IRRH rates were 10.8, 6.1, 6.4, 10.8, 9.5, 9.7, 8.7, 12.3, and 10.7%, respectively. By the end of the study, 104 (16.7%) patients had IRRHs. Types of infection consisted of 36 (34.6%) lower respiratory tract infections, 33 (31.7%) soft tissue or muscular skeletal system infections, 16 (15.4%) urogenital tract infections, 10 (9.6%) gastrointestinal tract infections, and nine (8.7%) cases of sepsis. There were 94 (90.4%) bacterial, five (4.8%) viral, one (1%) fungal, and four (3.8%) uncertain types of infections diagnosed presumptively based on the clinical presentation, biomarkers, and images findings. Pathogens were identified in 44 (42.3%) patient, including bacteria in 40 (90.9%), viruses in three (6.8%), and fungus in one (2.2%) patients. Of the 104 patients who experienced IRRHs, the time from the index hospitalization to IRRH was 1.0 (0.4; 2.6) years.
Predictors of infection-related rehospitalization after decompensated heart failure
In Cox univariate analysis, predictors of IRRH included age, female sex, SBP, New York Heart Association functional class at least III, diabetes mellitus, hypertension, atrial fibrillation, chronic obstructive pulmonary disease, not taking ACEIs or ARBs, not taking beta blockers, MTLD, lower Hb levels, serum albumin levels, and estimated glomerular filtration rates (eGFRs) (Table 2). Cox multivariable analysis showed that age, diabetes mellitus, not taking ACEIs or ARBs, MTLD, lower Hb levels, and lower eGFRs were independently associated with IRRH. Kaplan–Meier curves reveal that patients who need MTLD had significantly lower IRRH event-free survival compared with those who didn’t need MTLD (P < 0.001) (Fig. 1a). Patients who didn’t take ACEIs or ARBs had significantly lower IRRH event-free survival compared with who took ACEIs or ARBs (P = 0.004) (Fig. 1b).
Table 2.
Univariate and multivariable analysis for predictors of infection-related rehospitalization n = 622
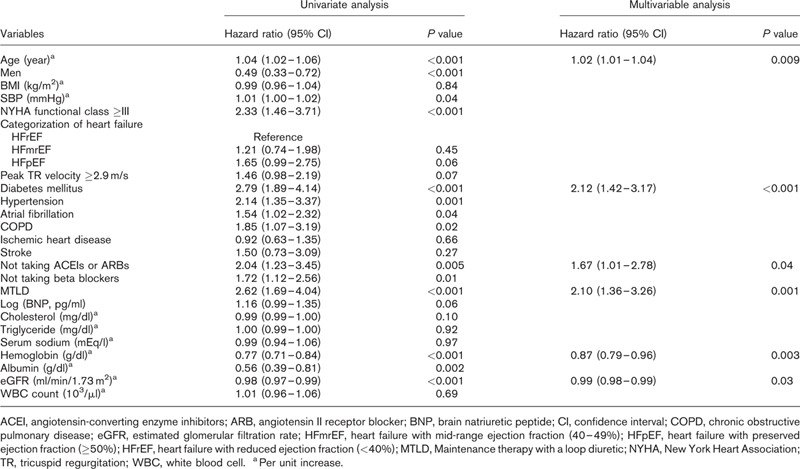
Fig. 1.
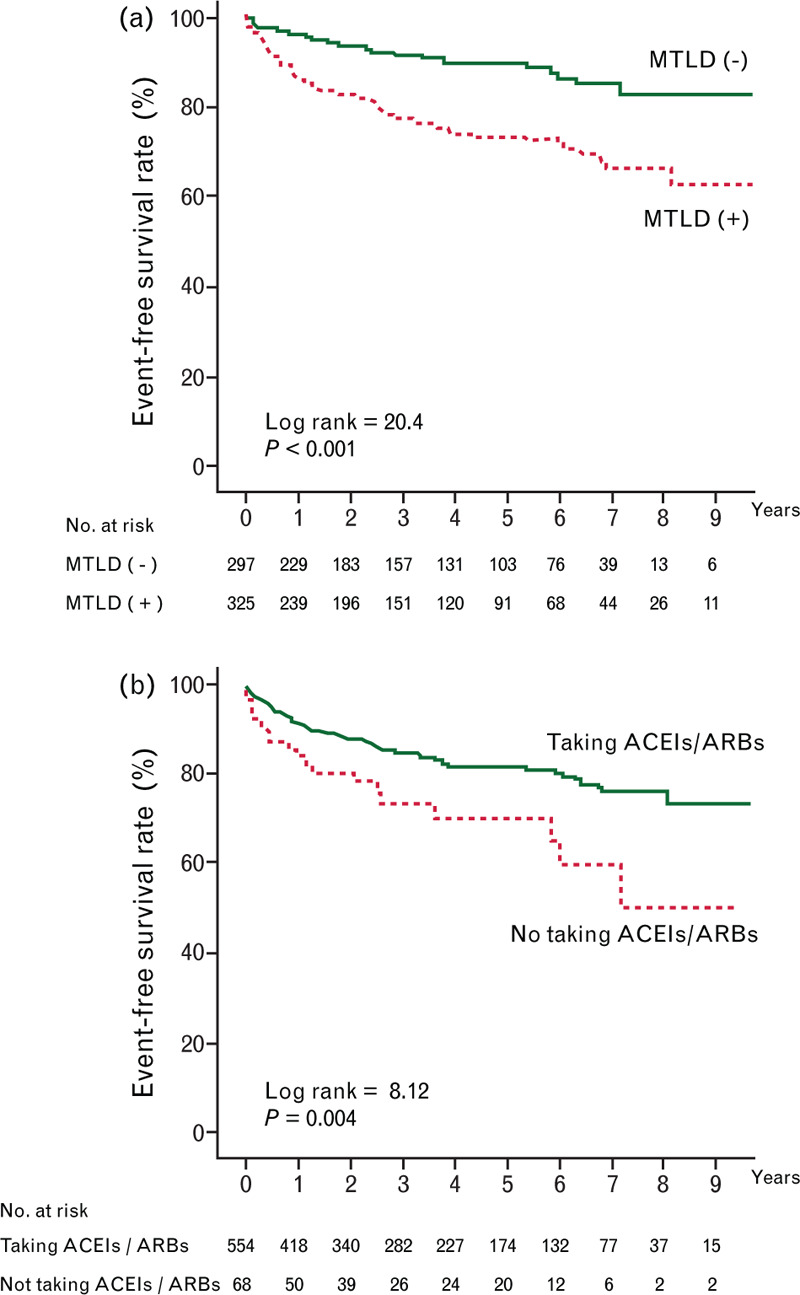
Kaplan–Meier curves for infection-related rehospitalization event-free survival among heart failure patients categorized by needing or not needing maintenance therapy with a loop diuretic (a) and taking or not taking angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers (b).
To predict IRRH, the area under the receiver operating characteristic curve of the Hb was 0.64. Based on Youden's index, the cutoff values for men and women patients were set at 13.2 and 12.5 g/dl, respectively. Men with Hb less than 13.2 g/dl and women with Hb less than 12.5 g/dl had significantly lower IRRH event-free survival compared with men with Hb at least 13.2 g/dl and women with Hb at least 12.5 g/dl (P < 0.001) (Fig. 2a).
Fig. 2.
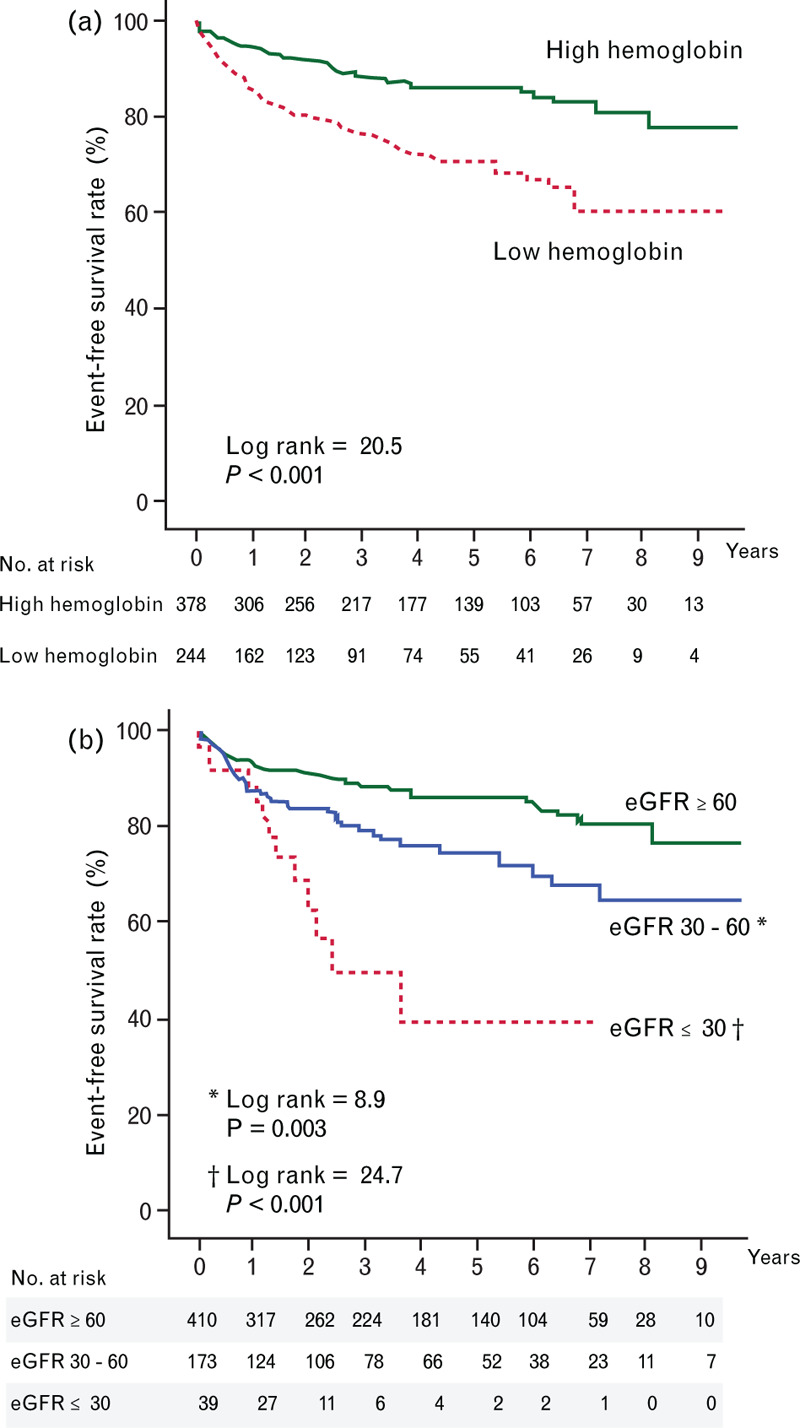
Kaplan–Meier curves for infection-related rehospitalization event-free survival in heart failure patients categorized by high hemoglobin (hemoglobin ≥13.2 and ≥12.5 g/dl for men and women, respectively) and low hemoglobin levels (hemoglobin <13.2 and <12.5 g/dl for men and women, respectively) (a), and by estimated glomerular filtration rates of at least 60, 30–60, and of 30 ml/min/1.73 m2 or less. ∗Log rank = 8.9, P = 0.003 compared with estimated glomerular filtration rates at least 60 ml/min/1.73 m2. †Log rank = 24.7, P < 0.001 compared with estimated glomerular filtration rates at least 60 ml/min/1.73 m2.
When patients were categorized by eGFRs of at least 60, 30–60 and 30 ml/min/1.73 m2 or less, lower eGFRs were associated with a graded increased risk of IRRH. Specifically, patients with eGFR 30–60 ml/min/1.73 m2 had a near doubled risk of IRRH compared with those with eGFR at least 60 ml/min/1.73 m2 (hazard ratio = 1.83, 95% CI = 1.20–2.77, P = 0.005). Moreover, patients with eGFR of 30 ml/min/1.73 m2 or less had a risk of IRRH more than four times higher than those with eGFR at least 60 ml/min/1.73 m2 (hazard ratio = 4.18, 95% CI = 2.26–7.73, P < 0.001) (Fig. 2b).
The impact of infection-related rehospitalization on long-term survival
Overall, 24.4% (n = 152) of the 622 patients had died by the end of the study. Of the 104 patients who experienced IRRH, 41.3% (n = 43) had died, whereas only 21% (n = 109) of the 518 patients who did not experience IRRH had died. Of the 43 patients who experienced IRRH and had died by the end of the study, the time from IRRH to death was 0.6 (0.1; 2.7) years. When compared with patients who didn’t experience IRRH, patients who experienced IRRH were associated with an increased risk of death during follow-up (hazard ratio = 2.79, 95% CI = 1.96–3.97, P < 0.001). IRRH was an independent predictor of all-cause mortality (hazard ratio = 1.99, 95% CI = 1.32–2.98, P = 0.001) after adjusting for the parameters well documented in previous studies13,14 and identified in our univariate analysis, including age, BMI, New York Heart Association functional class, chronic obstructive pulmonary disease, brain natriuretic peptide, Hb, and eGFR (Supplemental Table). The increased risk of death was primarily linked to IRRHs for lower respiratory tract infections (hazard ratio = 3.71, 95% CI = 2.28–6.04, P < 0.001), urogenital tract infections (hazard ratio = 2.83, 95% CI = 1.32–6.10, P = 0.008), and sepsis (hazard ratio = 3.26, 95% CI = 1.20–8.85, P = 0.020) (Fig. 3).
Fig. 3.
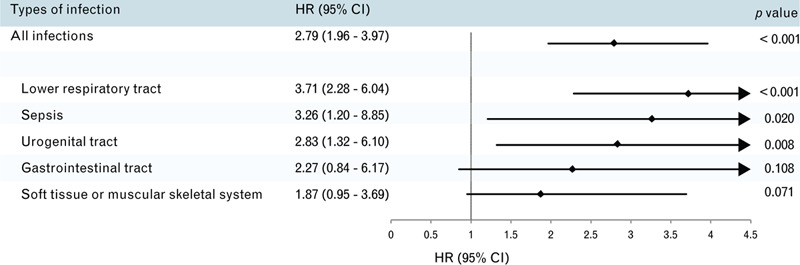
Forest plot portraying the hazard ratios and 95% confidence intervals of the association between the risk of long-term all-cause mortality after infection-related rehospitalization, categorized by sepsis, lower respiratory tract, urogenital tract, gastrointestinal tract, and soft tissue or muscular skeletal system infections.
Discussion
To the best of our knowledge, this is the first prospective study investigating predictors of IRRH in patients discharged for acute decompensated heart failure and its impact on long-term survival. Our main findings were: first, one out of six heart failure patients experienced at least one IRRH after the index hospitalization for decompensated heart failure. IRRH rates were approximately 10% (6.1–12.3%) each year after discharge from hospital for decompensated heart failure; second, IRRH independently predicted long-term all-cause mortality. The increased risk of death was predominantly related to lower respiratory tract infections, urogenital tract infections, and sepsis; third, independent predictors of IRRH after acute decompensated heart failure were old age, diabetes mellitus, not taking ACEIs or ARBs, MTLD, lower Hb levels, and lower eGFR.
It is well known that cardiovascular disease-related hospitalizations for heart failure patients are associated with worse subsequent survival.3,4,15 However, rare study has addressed the impact of infection-related hospitalization on long-term survival in heart failure patients. Previously, Alon et al. showed an increased 30-day mortality rate among heart failure patients hospitalized for infection compared with heart failure patients hospitalized for other reasons using a retrospective study design on the basis of the International Classification of Disease-9 diagnosis codes. They showed that the higher mortality rate was primarily related to respiratory infections, bacteremia, and sepsis.16 Our findings extended the impact of IRRH on survival from 30 days (shown by Alon et al.) to 3.9 ± 2.7 years (in our study). In addition to the adverse cardiovascular impact of acute infection,17 infection-induced inflammatory responses can cause chronic atherogenesis, myocardial fibrosis, and adverse myocardial remodeling.18 These mechanisms might explain the impact of IRRH on long-term survival in heart failure patients.
It is possible that frail patients with serious underlying medical conditions were more commonly rehospitalized due to IRRH. Consequently, these frail patients had a higher mortality rate. To clarify this issue, we conducted a multivariable analysis to see whether IRRH played an independent role in predicting mortality after adjusting for factors probably related to frailty. From the literature, we learned that age,19 BMI,20 New York Heart Association functional class,21 chronic obstructive pulmonary disease,22 brain natriuretic peptide,23 Hb,24 and eGFR25 were associated with frailty. Based on recruiting all these factors in the multivariable analysis model, we demonstrated that IRRH independently predicted all-cause mortality (Supplement Table). It suggests that the increased risk of death associated with IRRHs could not be completely explained by frailty. However, since our initial study design was not for frailty, frailty assessment instruments such as the frailty phenotype and frailty index26 were not included in this study. Therefore, our results cannot completely exclude the possibility of association between frailty and mortality. Further studies are needed to shed light on this issue.
The associations of old age and diabetes mellitus with increased risk of infection have been reported in both heart failure16 and nonheart failure patients.27,28 In addition to old age and diabetes mellitus, interesting factors meriting further investigation include MTLD, not taking ACEIs or ARBs, lower Hb levels, and lower eGFRs.
Maintenance therapy with a loop diuretic as a predictor of infection-related rehospitalization
Even with our multidisciplinary disease management program directing patients to restrict intake of salt and fluid, half of our patients still needed daily diuretics to control symptoms and signs related to fluid overload. We found patients who needed MTLD were associated with increased risk of subsequent IRRH. The mechanisms are not entirely clear. There have been reports mentioning that edema in heart failure patients is associated with altered alveolar capillary barrier and higher gut permeability, bacterial translocation in the gut and higher concentrations of inflammatory cytokines.29,30 Prior studies have also shown that clinical signs of edema are associated with worse survival.31 However, no previous study has examined the relation of IRRH with edema and the need to take diuretics. Our study shows that the effect of refractory edema on patient outcomes is much greater than we thought. The chain reactions ensuing from edema, changes in gut and alveolar capillary barrier permeability, to subsequent infection and/or inflammation are potential therapeutic targets for preventing IRRH and deterioration of heart failure.
Not taking angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers as a predictor of infection-related rehospitalization
ACEIs and ARBs are well known medications that reduce mortality and morbidity by regulating the renin–angiotensin system in heart failure patients.10 In additional to the traditional role of regulating vascular resistance and fluid and electrolyte balance, there are increasing evidences that renin–angiotensin system is involved in the inflammatory response.32 Previous studies have shown reduced systemic inflammation in heart failure patients taking ACEIs33 and in hypertension patients taking ARBs.34,35 An association between using ACEIs and reduced risk of pneumonia has also been reported.36,37 So far, no previous study has focused on the relation between IRRHs and use of ACEIs or ARBs. Our results show that in addition to their traditional cardiovascular protective effects, use of ACEIs or ARBs was associated with a lower rate of IRRHs.
Lower hemoglobin levels as a predictor of infection-related rehospitalization
Previous studies have shown that anemia in heart failure patients is not only associated with renal dysfunction, iron deficiency, decreased bone marrow erythropoiesis, but also is a marker of inflammatory response.38 The elevated inflammatory response is associated with high risk of infection.39 Though researchers have shown that anemia is an adverse prognostic indicator in heart failure patients,40,41 rare prior studies have focused on the level of Hb as a predictor of IRRH among heart failure patients. In line with our findings, Alon et al.16 also found a relationship between lower Hb levels and IRRH. Based on a prospective design, we demonstrated that the risk of IRRH increased by 13% per unit (g/dl) decrease of Hb. We further demonstrated that men with Hb less than 13.2 g/dl and women with Hb less than 12.5 g/dl had significantly lower IRRH event-free survival compared with men with Hb at least 13.2 g/dl and women with Hb at least 12.5 g/dl. The cutoff values of 13.2 g/dl for men and 12.5 g/dl for women in this cohort are close to the anemia definition of 2016 European Society of Cardiology guidelines for heart failure (Hb <13 g/dl in men and <12 g/dl in women).10 Further investigations to explore the function of bone marrow and erythropoiesis are mandatory for tackling IRRH.
Lower estimated glomerular filtration rate as a predictor of infection-related rehospitalization
Many heart failure patients have chronic kidney disease (CKD), which has a reported incidence of 32%.42 Infection is a common cause of hospitalization and mortality in patients with CKD.43 It is necessary to identify heart failure patients with CKD who are at high-risk for IRRH for further care planning. Consistent with our findings, one previous study has shown that lower eGFR was associated with a graded increased risk of all-cause hospitalizations in heart failure patients with CKD.44 Our results highlight the need to develop effective interventions to prevent IRRH among heart failure patients with advanced CKD.
Study limitations
The current study has a few limitations. First, this is a single hospital study on the basis of the multidisciplinary disease management program with a longitudinal follow-up study design for heart failure patients enrolled at our heart failure center. The relatively small sample size limits the power of the study. The results presented here should be confirmed by prospective multicenter studies with long-term follow-up. Second, the mean age 59 years in our study is younger than that of heart failure patients in the Western countries. However, one previous community-based prospective cohort study of investigating heart failure prognosis in Taiwan showed that the average age of heart failure patients was around 58 years.45 The younger age in our patients is probably due to different ethnicities. Finally, parameters regarding inflammation should be fully investigated in the future.
Conclusion
The rates of IRRH remained as high as 10% each year over the 10 years following hospitalization for acute decompensated heart failure. IRRH independently predicted long-term all-cause mortality. We found that independent predictors of IRRH after discharge from acute decompensated heart failure were old age, diabetes mellitus, not taking ACEIs or ARBs, MTLD, lower Hb levels, and lower eGFRs. These findings warrant future study to tackle the problem of IRRH.
Acknowledgements
The authors thank Cardiology Section, Department of Internal Medicine, Chang Gung Memorial Hospital, Keelung, Taiwan for providing samples from patients. We also thank Bing-Yu Chen and Tay-Wey Lee of the Medical Research Center at Chang Gung Memorial Hospital in Keelung, Taiwan, R.O.C. for their statistical assistance.
The current study was supported in part by the Ministry of Science and Technology of Taiwan (MOST107-2314-B-182-071-MY2), and Chang Gung Memorial Hospital (CMRPG2G0601, 2G0591, CORPG 2J0021, 2J0011).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
References
- 1.Ambrosy AP, Fonarow GC, Butler J, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014; 63:1123–1133. [DOI] [PubMed] [Google Scholar]
- 2.Ni H, Xu J. Recent trends in heart failure-related mortality: United States, 2000–2014. NCHS data brief 2015; 1–8. [PubMed] [Google Scholar]
- 3.Solomon SD, Dobson J, Pocock S, et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation 2007; 116:1482–1487. [DOI] [PubMed] [Google Scholar]
- 4.Pocock SJ, Wang D, Pfeffer MA, et al. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J 2006; 27:65–75. [DOI] [PubMed] [Google Scholar]
- 5.Dunlay SM, Redfield MM, Weston SA, et al. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol 2009; 54:1695–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao CT, Liu MH, Hsu KH, et al. Effect of multidisciplinary disease management for hospitalized heart failure under a national health insurance programme. J Cardiovasc Med (Hagerstown) 2015; 16:616–624. [DOI] [PubMed] [Google Scholar]
- 7.Battler A, Karliner JS, Higgins CB, et al. The initial chest x-ray in acute myocardial infarction. Prediction of early and late mortality and survival. Circulation 1980; 61:1004–1009. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell C, Rahko PS, Blauwet LA, et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 2019; 32:1–64. [DOI] [PubMed] [Google Scholar]
- 9.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37:67–119. [DOI] [PubMed] [Google Scholar]
- 10.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 11.Mullens W, Damman K, Harjola VP, et al. The use of diuretics in heart failure with congestion – a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019; 21:137–155. [DOI] [PubMed] [Google Scholar]
- 12.Altman DG, De Stavola BL. Practical problems in fitting a proportional hazards model to data with updated measurements of the covariates. Stat Med 1994; 13:301–341. [DOI] [PubMed] [Google Scholar]
- 13.Barlera S, Tavazzi L, Franzosi MG, et al. Predictors of mortality in 6975 patients with chronic heart failure in the Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto Miocardico-Heart Failure trial: proposal for a nomogram. Circ Heart Fail 2013; 6:31–39. [DOI] [PubMed] [Google Scholar]
- 14.Rahimi K, Bennett D, Conrad N, et al. Risk prediction in patients with heart failure: a systematic review and analysis. JACC Heart Fail 2014; 2:440–446. [DOI] [PubMed] [Google Scholar]
- 15.Chun S, Tu JV, Wijeysundera HC, et al. Lifetime analysis of hospitalizations and survival of patients newly admitted with heart failure. Circ Heart Fail 2012; 5:414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alon D, Stein GY, Korenfeld R, Fuchs S. Predictors and outcomes of infection-related hospital admissions of heart failure patients. PLoS One 2013; 8:e72476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin L, Derwall M, Al Zoubi S, et al. The septic heart: current understanding of molecular mechanisms and clinical implications. Chest 2019; 155:427–437. [DOI] [PubMed] [Google Scholar]
- 18.Bhatt AS, DeVore AD, Hernandez AF, Mentz RJ. Can vaccinations improve heart failure outcomes? There are contemporary data and future directions. JACC Heart Fail 2017; 5:194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 2018; 15:505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferriolli E, Pessanha FPADS, Moreira VG, Dias RC, Neri AL, Lourenco RA. Body composition and frailty profiles in Brazilian older people: Frailty in Brazilian Older People Study-FIBRA-BR. Arch Gerontol Geriatr 2017; 71:99–104. [DOI] [PubMed] [Google Scholar]
- 21.Yaku H, Kato T, Morimoto T, et al. Risk factors and clinical outcomes of functional decline during hospitalisation in very old patients with acute decompensated heart failure: an observational study. BMJ Open 2020; 10:e032674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanlon P, Nicholl BI, Jani BD, Lee D, McQueenie R, Mair FS. Frailty and prefrailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK Biobank participants. Lancet Public Health 2018; 3:e323–e332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishiguchi S, Nozaki Y, Yamaji M, et al. Plasma brain natriuretic peptide level in older outpatients with heart failure is associated with physical frailty, especially with the slowness domain. J Geriatr Cardiol 2016; 13:608–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirani V, Naganathan V, Blyth F, et al. Cross-sectional and longitudinal associations between anemia and frailty in older Australian men: the concord health and aging in men project. J Am Med Dir Assoc 2015; 16:614–620. [DOI] [PubMed] [Google Scholar]
- 25.Chowdhury R, Peel NM, Krosch M, Hubbard RE. Frailty and chronic kidney disease: a systematic review. Arch Gerontol Geriatr 2017; 68:135–142. [DOI] [PubMed] [Google Scholar]
- 26.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet 2019; 394:1365–1375. [DOI] [PubMed] [Google Scholar]
- 27.Kline KA, Bowdish DM. Infection in an aging population. Curr Opin Microbiol 2016; 29:63–67. [DOI] [PubMed] [Google Scholar]
- 28.Gallacher SJ, Thomson G, Fraser WD, Fisher BM, Gemmell CG, MacCuish AC. Neutrophil bactericidal function in diabetes mellitus: evidence for association with blood glucose control. Diabet Med 1995; 12:916–920. [DOI] [PubMed] [Google Scholar]
- 29.Pappas L, Filippatos G. Pulmonary congestion in acute heart failure: from hemodynamics to lung injury and barrier dysfunction. Rev Esp Cardiol 2011; 64:735–738. [DOI] [PubMed] [Google Scholar]
- 30.Niebauer J, Volk HD, Kemp M, et al. Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet 1999; 353:1838–1842. [DOI] [PubMed] [Google Scholar]
- 31.Damy T, Kallvikbacka-Bennett A, Zhang J, et al. Does the physical examination still have a role in patients with suspected heart failure? Eur J Heart Fail 2011; 13:1340–1348. [DOI] [PubMed] [Google Scholar]
- 32.Ranjbar R, Shafiee M, Hesari A, Ferns GA, Ghasemi F, Avan A. The potential therapeutic use of renin–angiotensin system inhibitors in the treatment of inflammatory diseases. J Cell Physiol 2019; 234:2277–2295. [DOI] [PubMed] [Google Scholar]
- 33.Gullestad L, Aukrust P, Ueland T, et al. Effect of high- versus low-dose angiotensin converting enzyme inhibition on cytokine levels in chronic heart failure. J Am Coll Cardiol 1999; 34:2061–2067. [DOI] [PubMed] [Google Scholar]
- 34.Fliser D, Buchholz K, Haller H. Antiinflammatory effects of angiotensin II subtype 1 receptor blockade in hypertensive patients with microinflammation. Circulation 2004; 110:1103–1107. [DOI] [PubMed] [Google Scholar]
- 35.Manabe S, Okura T, Watanabe S, Fukuoka T, Higaki J. Effects of angiotensin II receptor blockade with valsartan on pro-inflammatory cytokines in patients with essential hypertension. J Cardiovasc Pharmacol 2005; 46:735–739. [DOI] [PubMed] [Google Scholar]
- 36.Ohkubo T, Chapman N, Neal B, Woodward M, Omae T, Chalmers J. Effects of an angiotensin-converting enzyme inhibitor-based regimen on pneumonia risk. Am J Respir Crit Care Med 2004; 169:1041–1045. [DOI] [PubMed] [Google Scholar]
- 37.Okaishi K, Morimoto S, Fukuo K, et al. Reduction of risk of pneumonia associated with use of angiotensin I converting enzyme inhibitors in elderly inpatients. Am J Hypertens 1999; 12:778–783. [DOI] [PubMed] [Google Scholar]
- 38.O’Meara E, Rouleau JL, White M, et al. Heart failure with anemia: novel findings on the roles of renal disease, interleukins, and specific left ventricular remodeling processes. Circ Heart Fail 2014; 7:773–781. [DOI] [PubMed] [Google Scholar]
- 39.Hasper D, Hummel M, Kleber FX, Reindl I, Volk HD. Systemic inflammation in patients with heart failure. Eur Heart J 1998; 19:761–765. [DOI] [PubMed] [Google Scholar]
- 40.O’Meara E, Clayton T, McEntegart MB, et al. Clinical correlates and consequences of anemia in a broad spectrum of patients with heart failure: results of the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Program. Circulation 2006; 113:986–994. [DOI] [PubMed] [Google Scholar]
- 41.Anand IS, Kuskowski MA, Rector TS, et al. Anemia and change in hemoglobin over time related to mortality and morbidity in patients with chronic heart failure: results from Val-HeFT. Circulation 2005; 112:1121–1127. [DOI] [PubMed] [Google Scholar]
- 42.Damman K, Valente MA, Voors AA, O’Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J 2014; 35:455–469. [DOI] [PubMed] [Google Scholar]
- 43.Dalrymple LS, Go AS. Epidemiology of acute infections among patients with chronic kidney disease. Clin J Am Soc Nephrol 2008; 3:1487–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith DH, Thorp ML, Gurwitz JH, et al. Chronic kidney disease and outcomes in heart failure with preserved versus reduced ejection fraction: the Cardiovascular Research Network PRESERVE Study. Circ Cardiovasc Qual Outcomes 2013; 6:333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang CH, Chien KL, Chen WJ, et al. Impact of heart failure and left ventricular function on long-term survival – report of a community-based cohort study in Taiwan. Eur J Heart Fail 2007; 9:587–593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


