Figure 1.
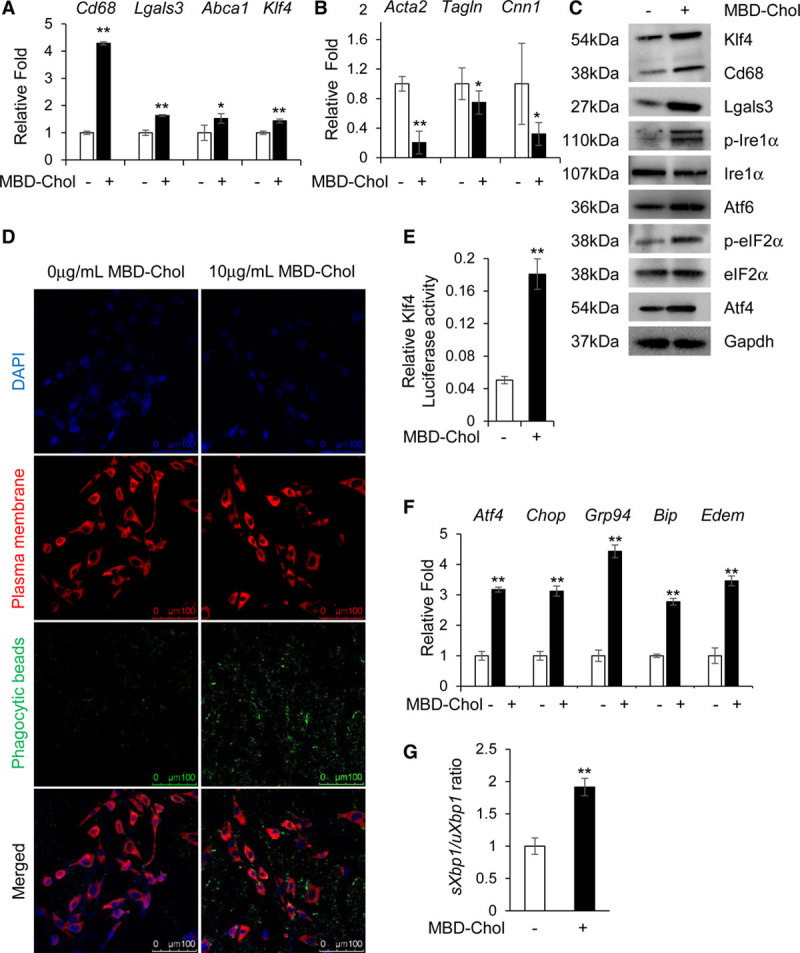
Cholesterol induces phenotypic switching and endoplasmic reticulum (ER) stress in immortalized vascular smooth muscle cells (SMCs). A and B, With exposure to 10 µg/mL methyl-β-cyclodextrin cholesterol (MBD-Chol) for 72 h, expression of macrophage markers Cd68 and Lgals3 along with Abca1 and Klf4 (Krüppel-like factor 4) are upregulated (A), while contractile marker genes Acta2, Tagln and Cnn1 are downregulated (B). C, Immunoblots confirm increased protein levels of Cd68 (cluster of differentiation 68), Lgals3 (lectin, galactoside-binding, soluble, 3), and Klf4 along with induction of ER stress including phosphorylation of Ire (inositol-requiring enzyme) 1α and eIF2α (α-subunit of the eukaryotic elongation factor 2), induction of Atf (activating transcription factor) 4 and increased cleavage of Atf6. D, Exposure to 10 µg/mL MBD-Chol for 72 h increases phagocytic activity of SMCs as evident from the increased uptake of green fluorescent phagocytic beads. E, Klf4 transcriptional activity is increased when SMCs are exposed to cholesterol. F, Cholesterol exposure upregulates ER stress effector genes like Atf4 and Chop as well as the chaperones Grp94, Bip, and Edem. G, Cholesterol treatment increases splicing of Xbp1, indicating activation of the Ire1α pathway. Each result displayed here is representative of at least 3 independent biological replicates. P values were calculated using unpaired 2-tailed Student t test. *P<0.05 or **P<0.01, vs no cholesterol treatment. DAPI indicates 4′,6-diamidino-2-phenylindole; p-eIF2α, phosphorylated eIF2α; and p-Ire1α, phosphorylated Ire1α.
