Psoriasis is a common chronic recurrent inflammatory disease. Plaque psoriasis accounts for more than 90% of psoriasis patients. Currently, no therapies are curative. Biologics have greatly improved the clinical efficacy and potentially provide a better long-term safety profile, with respect to toxicity to major organs, than conventional treatments; however, relapse after treatment withdrawal is still an important issue. Although maintenance treatment is recommended in most guidelines, treatment withdrawal occurs quite frequently in real-world clinical practice. Thus, the time to relapse is an important consideration when choosing among the different therapies.
We analyzed the available literature with respect to biologic-based strategies for psoriasis treatment and relapse time after withdrawal. The selected biologics, including their approval dates, standard regimen, and half-life time values are listed in Supplementary Table 1. Two head-to-head studies and fourteen individual studies with relapse data were included. The definition of “relapse” varied among the different studies, the most common being “loss of a 50% reduction in the Psoriasis Area and Severity Index (PASI 50) and a Physician's Global Assessment score (PGA) ≥ 3”. The comparison of relapse time among different biologics with the same relapse definition is presented in Figure 1.
Figure 1.
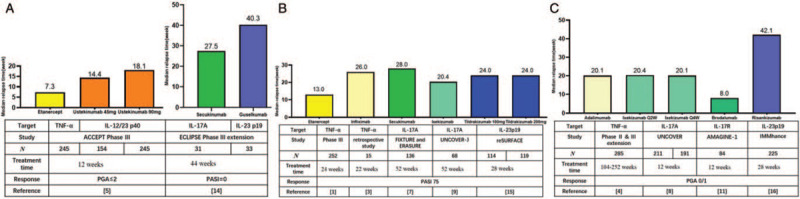
Comparison of the median time to relapse among different biologics. (A) Head-to-head comparison of time to relapse (PGA ≥ 3) between etanercept and ustekinumab (left); head-to-head comparison of time to relapse (initiation of new systemic treatment) between secukinumab and guselkumab (right). (B) Time to relapse (loss of PASI 50) in individual studies. (C) Time to relapse (PGA ≥ 3) in individual studies. IL: Interleukin; PASI: Psoriasis Area and Severity Index; PGA: Physician's Global Assessment score; TNF: Tumor necrosis factor.
Tumor necrosis factor (TNF) inhibitors
Etanercept
Among 409 patients who were assigned to stop etanercept treatment at week 24, 347 relapsed (showing loss of PASI 50) within the 24-week period. The median time to relapse was 85 days (range: 70–91 days) in PASI 50 responders and 91 days (range: 85–112 days) in PASI 75 responders (252 patients).[1] Bellinato et al[2] reported that among 53 patients who were asked to discontinue etanercept after achieving a stable clinical remission (PASI 0/1 for at least 1 year), 33 (62%) of the patients relapsed (PASI score variation ≥ 5) within 6 months. The median time to relapse was 184 days.
Infliximab
Giunta et al[3] presented a retrospective infliximab withdrawal study. In 15 patients (13 reached PASI 75) who received infliximab for 22 weeks and then discontinued, the median relapse time to loss of PASI 50 was 182 days (range: 84–308 days).
Adalimumab
Among 285 patients who had achieved PGA 0/1 and then stopped adalimumab treatment for up to 40 weeks, 178 relapsed (PGA ≥ 3), with the median time of 141 days (interquartile range [IQR]: 93–202 days).[4]
Interleukin (IL)-12/IL-23 inhibitors
Ustekinumab
Griffiths et al[5] conducted the ACCEPT trial, which included 903 patients who were randomized to receive ustekinumab (45 mg or 90 mg) or etanercept (50 mg). At week 12, patients with PGA ≤ 2 stopped treatment until psoriasis relapsed (PGA ≥ 3). The median time to relapse was 14.4, 18.1, and 7.3 weeks in the three groups, respectively. An 8-year multicenter retrospective study included 202 patients who had responded to ustekinumab treatment and then withdrawn.[6] The duration of ustekinumab treatment prior to withdrawal was 16.7 ± 10.2 months. The cumulative probability of relapse free was 49.3%, 12.6%, 5.3%, 4.7%, and 1.6% after 6, 12, 18, 24, and 36 months, respectively. The relapse time was 6.7 ± 4.1 months (range: 3–30.8 months). The authors of the study also tried to identify predictors and found the following possible predictors for a longer time to relapse: biologic-naïve, higher PASI improvement, shorter time to achieve PASI 50, no family history of psoriasis, no chronic kidney disease, and immunosuppressant use while ustekinumab was withdrawn.
IL-17 inhibitors
Secukinumab
Blauvelt et al[7] presented relapse results after secukinumab withdrawal. Among 181 patients who completed treatment with secukinumab (300 mg) for 52 weeks and then randomized to treatment withdrawal, the median time to loss of PASI 50 was 28 weeks (95% CI: 24.14–32.00) and the mean PASI at recurrence was 15.6 (range: 4.5–65.1).
Ixekizumab
Blauvelt et al[8] analyzed data pooled from two clinical trials. After the induction period of 12 weeks, 402 ixekizumab responders (PGA = 0/1) were randomized to placebo simulation. The median time to relapse (PGA ≥ 3) was 20.4 weeks in the ixekizumab Q2W/placebo group and 20.1 weeks in the ixekizumab Q4W/placebo group. Umezawa et al[9] reported that 70 patients who reached PASI 75 after 52 weeks of treatment were assigned to stop until relapse (loss of PASI 50). After withdrawal, approximately half of the subjects relapsed within 5 months. The median time to relapse was 143 days.
Brodalumab
Regnault et al[10] published a retrospective multicenter cohort study on brodalumab withdrawal. At the time of withdrawal, the mean duration of treatment was 3.1 ± 1.5 years. In total, 67 (87%) patients had reached PASI 90, while 74 (96%) had reached PASI 75. The relapse was defined by the desire of the patient to start a new treatment (topical or systemic) for psoriasis. Within the 9-month follow-up period, all of the patients relapsed; the median recurrence time was 46 days (range: 7–224 days). Papp et al[11] reported that 84 patients who attained PGA 0/1 with brodalumab (210 mg Q2W) were randomized to placebo simulation at week 12. The median time to relapse was 56.0 days.
IL-23 inhibitors
Guselkumab
As reported by Reich et al,[12] among 182 patients who had achieved PASI 90 at week 28 and then randomized to placebo simulation, the median time to loss of PASI 90 response was 15.2 weeks (23 weeks after last guselkumab dose); Gordon et al[13] further showed that 30.6% of patients experienced loss of PASI 50 by week 52, 49.1% by week 60, and 67.6% by week 72; although 32.4% showed no loss in PASI 50 by week 72. It was found that the correlation of IL-23 signaling serum cytokines (IL-17A, IL-17F, and IL-22) increased with disease recurrence; however, these increases had poor predictive value for relapse as they lagged after increases in PASI scores. Rivera et al[14] reported the head-to-head comparison of guselkumab and secukinumab withdrawal in Spanish patients who participated in the ECLIPSE study. The median time from the last dose of biologics to the initiation of new systemic treatment (relapse) was 282 days (IQR: 180–333 days) for patients with guselkumab and 192.5 days (IQR: 107–308 days) for patients with secukinumab. The univariate regression models revealed statistically significant differences in factors of arthritis and the psoriasis duration. Relapse time was longer in patients without arthritis or with a more recent diagnosis of psoriasis. The authors stated that these results might suggest that there is a window of opportunity in the treatment of psoriasis during which the natural course of the disease could be altered. Multivariate linear regression model adjusted for different covariates confirmed the association between the study drug and relapse time.
Tildrakizumab
Kimball et al[15] published relapse results after 28 weeks of tildrakizumab treatment (placebo simulation thereafter). The median time to loss of PASI 50 was 24 weeks in the group treated with 100 mg tildrakizumab (n = 114) as well as the group treated with 200 mg of this biologic (n = 119). The proportions of patients who experienced relapse were 0.9% (4 weeks after withdrawal), 5.5% (12 weeks), 10.8% (24 weeks), and 11.8% (36 weeks) in the 100 mg treatment group and 0.8%, 3.5%, 14.1%, and 10.0% at each corresponding time point in the 200-mg group.
Risankizumab
Among 225 patients who stopped risankizumab treatment at week 28 (placebo simulation thereafter), PGA 0/1 could be maintained in 61.3% at week 52 and 7.1% at week 104. The median time to relapse (PGA ≥ 3) was 295 days (IQR: 211–428 days).[16]
Conclusion
Based on this review of published studies, we conclude that the time to relapse after psoriasis treatment withdrawal varies among different biologics, partly due to the differences in their mechanisms of action and pharmacokinetics of specific drugs. IL inhibitors, especially IL-23 inhibitors, generally sustained longer time to relapse after treatment withdrawal than TNF inhibitors. Possible clinical predictors for longer relapse time, as reported in the literature, are as follows: biologic-naive, shorter psoriasis duration, no arthritis or chronic kidney disease, no family history of psoriasis, and higher and more rapid response to treatment.
Acknowledgements
The authors thank Hua Zhang from Research Center of Clinical Epidemiology, Peking University Third Hospital, for helpful discussions.
Funding
This work was supported by a grant from the 2019 Grant of Milstein Medical Asian American Partnership Foundation.
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Wang XY, Zhang CL, Wang WH. Time to relapse after treatment withdrawal for different biologics used to treat plaque psoriasis. Chin Med J 2020;133:2998–3000. doi: 10.1097/CM9.0000000000001232
Supplemental digital content is available for this article.
References
- 1.Gordon KB, Gottlieb AB, Leonardi CL, Elewski BE, Wang A, Jahreis A, et al. Clinical response in psoriasis patients discontinued from and then reinitiated on etanercept therapy. J Dermatolog Treat 2006; 17:9–17. doi: 10.1080/09546630500472838. [DOI] [PubMed] [Google Scholar]
- 2.Bellinato F, Girolomoni G, Gisondi P. Relapse of psoriasis in patients who asked to discontinue etanercept after achieving a stable clinical remission. Br J Dermatol 2019; 181:1319–1320. doi: 10.1111/bjd.18225. [DOI] [PubMed] [Google Scholar]
- 3.Efficacy and safety of infliximab in long-term treatment of plaque-type psoriasis: A comparative retrospective study on two different (continuous vs. relapse related) regimens. J Am Acad Dermatol 2007; 56:B190.doi: 10.1016/j.jaad.2006.10.866. [Google Scholar]
- 4.Papp K, Crowley J, Ortonne JP, Leu J, Okun M, Gupta SR, et al. Adalimumab for moderate to severe chronic plaque psoriasis: efficacy and safety of retreatment and disease recurrence following withdrawal from therapy. Br J Dermatol 2011; 164:434–441. doi: 10.1111/j.1365-2133.2010.10139.x. [DOI] [PubMed] [Google Scholar]
- 5.Griffiths CE, Strober BE, van de Kerkhof P, Ho V, Fidelus-Gort R, Yeilding N, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med 2010; 362:118–128. doi: 10.1056/NEJMoa0810652. [DOI] [PubMed] [Google Scholar]
- 6.Chiu HY, Hui RC, Tsai TF, Chen YC, Chang LN, Chen PH, et al. Predictors of time to relapse following ustekinumab withdrawal in patients with psoriasis who had responded to therapy: An eight-year multicenter study. J Am Acad Dermatol 2019; doi: 10.1016/j.jaad.2019.01.035. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 7.Blauvelt A, Reich K, Warren RB, Szepietowski JC, Sigurgeirsson B, Tyring SK, et al. Secukinumab re-initiation achieves regain of high response levels in patients who interrupt treatment for moderate to severe plaque psoriasis. Br J Dermatol 2017; 177:879–881. doi: 10.1111/bjd.15656. [DOI] [PubMed] [Google Scholar]
- 8.Blauvelt A, Papp KA, Sofen H, Augustin M, Yosipovitch G, Katoh N, et al. Continuous dosing versus interrupted therapy with ixekizumab: an integrated analysis of two phase 3 trials in psoriasis. J Eur Acad Dermatol Venereol 2017; 31:1004–1013. doi: 10.1111/jdv.14163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Umezawa Y, Torisu-Itakura H, Morisaki Y, ElMaraghy H, Nakajo K, Akashi N, et al. Long-term efficacy and safety results from an open-label phase III study (UNCOVER-J) in Japanese plaque psoriasis patients: impact of treatment withdrawal and retreatment of ixekizumab. J Eur Acad Dermatol Venereol 2019; 33:568–576. doi: 10.1111/jdv.15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masson RM, Konstantinou MP, Khemis A, Poulin Y, Bourcier M, Amelot F, et al. Early relapse of psoriasis after stopping brodalumab: a retrospective cohort study in 77 patients. J Eur Acad Dermatol Venereol 2017; 31:1491–1496. doi: 10.1111/jdv.14387. [DOI] [PubMed] [Google Scholar]
- 11.Papp K, Menter A, Leonardi C, Soung J, Weiss S, Pillai R, et al. Long-term efficacy and safety of brodalumab in psoriasis through 120 weeks and after withdrawal and retreatment: subgroup analysis of a randomized phase III trial (AMAGINE-1). Br J Dermatol 2020; doi: 10.1111/bjd.19132. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reich K, Armstrong AW, Foley P, Song M, Wasfi Y, Randazzo B, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol 2017; 76:418–431. doi: 10.1016/j.jaad.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 13.Gordon KB, Armstrong AW, Foley P, Song M, Shen YK, Li S, et al. Guselkumab efficacy after withdrawal is associated with suppression of serum IL-23-regulated IL-17 and IL-22 in psoriasis: VOYAGE 2 study. J Invest Dermatol 2019; 139:2437–2446. doi: 10.1016/j.jid.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Rivera R, Martorell A, Lopez A, Salgado L, Sahuquillo A, de la Cueva P, et al. Maintenance of response following discontinuation of guselkumab and secukinumab in Spanish patients who participated in the ECLIPSE study. J Eur Acad Dermatol Venereol 2020; doi: 10.1111/jdv.16809. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15.Kimball AB, Papp KA, Reich K, Gooderham M, Li Q, Cichanowitz N, et al. Efficacy and safety of tildrakizumab for plaque psoriasis with continuous dosing, treatment interruption, dose adjustments and switching from etanercept: results from phase III studies. Br J Dermatol 2020; 182:1359–1368. doi: 10.1111/bjd.18484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blauvelt A, Leonardi CL, Gooderham M, Papp KA, Philipp S, Wu JJ, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol 2020; 156:649–658. doi: 10.1001/jamadermatol.2020.0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


