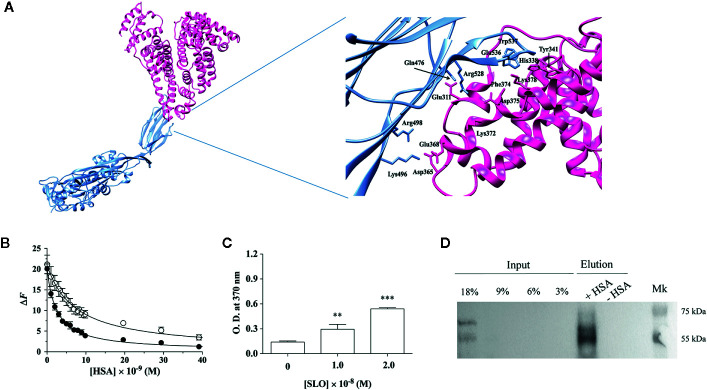
Figure 1.
HSA binds SLO toxin produced by Streptococcus pyogenes. (A) Molecular docking of SLO binding to HSA. Left: overall view of the putative complex between HSA (PDB ID: 1AO6) (deep pink) and SLO (PDB ID: 4HSC) (light blue) obtained by docking simulations. Right: residues involved in HSA-SLO interactions are highlighted. Images were drawn with the UCSF Chimera-package (35). (B) SLO binding to wt-HSA (filled circles) and to L305A/F374A-HSA (open circles) evaluated by spectrofluorimetric analysis. The continuous lines were calculated according to Eqn. 1 with the following parameters: wt-HSA - ΔF tot = 20.1 and K d = (2.5 ± 0.2)×10−9 M; and L305A/F374A-HSA - ΔF tot = 21.1 and K d = (7.3 ± 0.5)×10−9 M. Where not shown, the error bar is smaller than the symbol. (C) SLO binding to HSA evaluated by ELISA. Either 1.0×10−8 M or 2.0×10−8 M of activated SLO were added to HSA-coated wells, and the detection of HSA:SLO interaction was performed after incubation with anti-SLO antibody. Readings were performed using the TMB colorimetric substrate at 370 nm until reaction saturation. Microtiter wells coated with 100 μL of 1.6×10−7 M commercial HSA, without the subsequent addition of SLO, were used as a negative control. Results are representative of triplicates and are expressed as mean ± SD (One-way ANOVA, **P < 0.01 and ***P < 0.001). (D) SLO binding to recombinant wt-HSA evaluated by magnetic beads assay. Activated SLO (2.9×10−7 M) was incubated with 4 μg of wt-HSA-conjugated (+) or unconjugated (−) beads. Thirty microliters of magnetic beads eluates were resolved on a 12.5% SDS-PAGE and SLO bound to HSA was detected by immunoblot using anti-SLO antibody. Different quantities of SLO (i.e., 45, 90, 135, and 270 ng corresponding to 3%, 6%, 9%, and 18% of 1.5 μg SLO) were loaded as inputs. Results show the presence of the bands corresponding to SLO in the eluates.