Abstract
Background:
Kidney transplant recipients have higher risk of infectious diseases due to their reliance on immunosuppression. During the current COVID-19 pandemic, some clinicians might have opted for less potent immunosuppressive agents to counterbalance the novel infectious risk. We conducted a nationwide study to characterize immunosuppression use and subsequent clinical outcomes during the first 5 months of COVID-19 pandemic in the United States.
Methods:
Using data from the Scientific Registry of Transplant Recipients, we studied all kidney-only recipients in the United States from 1/1/2017 to 3/12/2020 (“prepandemic” era; n=64 849) and from 3/13/2020 to 7/31/2020 (“pandemic” era; n=5035). We compared the use of lymphocyte-depleting agents (vs. basiliximab or no induction) and maintenance steroids (vs. steroid avoidance/withdrawal) in the pandemic era compared to the prepandemic era. Then, we compared early posttransplant outcomes by immunosuppression regimen during the pandemic era.
Results:
Recipients in the pandemic era were substantially less likely to receive lymphocyte-depleting induction agents compared to their prepandemic counterparts (aOR=0.400.530.69); similar trends were found across subgroups of state-level COVID-19 incidence, donor type, and recipient age. However, lymphocyte-depleting induction agents were associated with decreased rejection during admission (aOR=0.110.230.47), but not with increased mortality in the pandemic era (aHR=0.130.471.66). On the other hand, the use of maintenance steroids versus early steroid withdrawal remained similar (aOR=0.711.071.62).
Conclusions:
The use of lymphocyte-depleting induction agents has decreased in favor of basiliximab and no induction during the COVID-19 pandemic. However, this shift might have resulted in increases in rejection with no clear reductions in posttransplant mortality.
INTRODUCTION
Immunosuppression is a vital part of posttransplant care that prevents rejections and prolongs graft survival.1–4 However, immunosuppression also increases the risk of infection, one of the leading causes of mortality in kidney transplant recipients.5,6 To balance such benefits and harms of immunosuppression, clinicians may tailor immunosuppression strategy based on the recipient’s risk profile.7,8 For example, milder immunosuppression strategies such as basiliximab-based induction immunosuppression and early steroid withdrawal are often considered for recipients with lower risk of rejection or higher risk of infection.9–11
During the current COVID-19 pandemic, the benefit-harm balance of immunosuppression might be altered because the potential harm of immunosuppression would be heightened by the risk of morbidity and mortality from COVID-19.12–14 In an attempt to counterbalance the novel infectious risk, some clinicians may opt for nonlymphocyte-depleting induction agents, such as basiliximab, or early steroid withdrawal for recipients who would have underwent lymphocyte-depleting induction immunosuppression or continued maintenance steroid had they been treated before the pandemic. The COVID-19 pandemic is expected to persist and seasonally cycle over the next several years.15,16 Given the central role of immunosuppression in regulating the risk of rejection and infection in kidney transplant recipients, it is crucial to identify the optimal strategy to counterbalance the novel infectious risk while effectively preventing rejection.
The first and urgent step is to characterize how transplant providers are adjusting their immunosuppression practices during this pandemic. As such, we conducted a national study examining the use of immunosuppressive agents and early clinical outcomes during the first 5 months of the pandemic. The goals of this study were to compare selection of induction and steroid-withdrawal protocols in the prepandemic versus pandemic eras, and to characterize early posttransplant outcomes by immunosuppression regimen among the recipients in the pandemic era.
MATERIALS AND METHODS
Data source
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donors, waitlisted candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), US Department of Health and Human Services, provides oversight to the activities of the OPTN and SRTR contractors. Additional details on the data can be found elsewhere.17
Study population
We studied all kidney-only transplant recipients in the United States from January 1, 2017 to July 31, 2020. We excluded 2756 (3.7%) recipients whose immunosuppression records were not available on the SRTR data, 578 (0.8%) recipients who remained hospitalized for more than 30 days posttransplant, and 536 (0.8%) recipients who died or had graft failure before discharge. The final study population included 69,884 recipients.
Immunosuppressive agents
The primary outcomes of this study were the use of lymphocyte-depleting agents for induction immunosuppression and steroids for maintenance immunosuppression at discharge from transplant admission. Lymphocyte-depleting agents included rabbit anti-thymocyte globulin, alemtuzumab, nonrabbit anti-thymocyte globulin, and rituximab. The use of lymphocyte-depleting agents was compared against the use of basiliximab, a combination of basiliximab and other agents, or no antibody-based induction. We defined maintenance steroids as the use of steroids at discharge from transplant admission, as opposed to compete avoidance or early steroid withdrawal before transplant discharge.4,18,19 Immunosuppression data were collected by the OPTN via the Immunosuppressive Medications form. The dose of the immunosuppressive agents is not available on the SRTR data and as such not analyzed in this study; however, as a surrogate marker, we examined the number of days anti-thymocyte globulin was administered.
Prepandemic era versus pandemic era
As the national emergency was declared on March 13, 2020,20 we divided the study period into the prepandemic era (January 1, 2017 – March 12, 2020) and the pandemic era (March 13, 2020 – July 31, 2020). We first compared the crude percentage of kidney transplant recipients in each induction agent category (anti-thymocyte globulin, alemtuzumab, other or multiple lymphocyte-depleting agents, basiliximab, basiliximab with other agents, and no antibody-based induction) and recipients with maintenance steroids during the prepandemic era versus the pandemic era.
We used logistic regression to test whether the tendency to use lymphocyte-depleting agents and maintenance steroids was different in the pandemic era compared to the prepandemic era. We implemented a stepwise model-building approach. Model 1 was based on univariable logistic regression with no adjustments. Model 2 included various recipient factors (age, race, sex, previous history of transplant, body mass index, diabetes, hypertension, primary cause of end-stage renal disease, time on dialysis, serum albumin, previous history of malignancy, previous history of peripheral vascular diseases, primary insurance, hepatitis C virus antibody, and cytomegalovirus antibody), donor factors (deceased vs. living, age, race, sex, body mass index, serum creatinine, hepatitis C virus antibody, cytomegalovirus antibody; for deceased donors – donation after circulatory death, diabetes, hypertension, stroke as the cause of donor death, pulsatile perfusion, and cold ischemic time; and for living donors – biologically related donor), and HLA-A/B/DR matching. Model 3 was our main analysis, based on multilevel logistic regression with random intercepts and random slopes. The random intercepts and slopes account for the variation in immunosuppression use across the transplant centers; specifically, the random intercept terms reflect the variation in each center’s overall tendency towards lymphocyte-depleting agents or maintenance steroids, and the random slope terms reflect the variation in the effect of the pandemic on each center’s immunosuppression use. Model 4 was a sensitivity analysis where we only included 172 (72.3%) centers that performed more than 3 recipients during the pandemic era. Observations with missing values were treated with missingness indicators.
Based on Model 3, we conducted subgroup analyses by state-level COVID-19 incidence (low, middle, or high), donor type (deceased or living), and recipient age (0-44, 45-64, or 65+ years). State-level COVID-19 incidences were calculated for 50 states and Washington D.C. using the cumulated number of cases as of March 13, 2020 as aggregated by the Center for Systems Science and Engineering at Johns Hopkins University21 and 2019 population estimates by the United States Census Bureau. States were divided into 3 groups based on the incidence; 10 states were assigned to the high-incidence subgroup (Washington, New York, Massachusetts, Washington D.C., Rhode Island, South Dakota, Colorado, Louisiana, California, and Oregon), 20 states were to the low-incidence subgroup (Arkansas, Indiana, Wyoming, Kansas, North Carolina, Michigan, Texas, Hawaii, Alaska, North Dakota, Arizona, Ohio, Alabama, Montana, Maine, Idaho, Oklahoma, Mississippi, Missouri, and West Virginia), and all other states were to the middle-incidence subgroup. We used interaction terms to examine if the change in the use of the immunosuppressive agents were statistically significantly different among the subgroups.
Early posttransplant outcomes
We assessed the association of lymphocyte-depleting agents (versus basiliximab or no induction) with acute rejection during transplant admission, delayed graft function, death-censored graft failure, and death, and the association of maintenance steroid (versus early steroid withdrawal) with death-censored graft failure and death. Acute rejection during admission and delayed graft function may occur prior to the selection of maintenance steroid versus early steroid withdrawal, and therefore were not studied as outcomes for maintenance steroid. All clinical outcomes were ascertained from the SRTR database. Acute rejection during transplant admission and delayed graft function were determined at the time of discharge and reported by the transplant centers to the OPTN/UNOS via the transplant recipient registration. Graft failure and death were actively reported by transplant centers and supplemented by OPTN/UNOS via linkage to the Social Security Death Master File17; our analyses included graft failures and deaths reported to OPTN/UNOS by July 31, 2020.
We conducted multilevel logistic regression for acute rejection and delayed graft function and stratified Cox regression for graft failure and death; these methods allow adjusting for center-level clustering. We used the inverse probability of treatment weights (IPTW) to adjust for potential confounders. IPTW were derived the Model 3 described above; we calculated the propensity score of receiving lymphocyte-depleting agents or maintenance steroid using the model and transformed it to IPTW. Recipients with extreme propensity scores (below the 1st or above the 99th percentile) were excluded from the analyses.
Statistical analysis
Confidence intervals are reported as per the method of Louis and Zeger.22 All analyses were performed using Stata 16.0/MP for Linux and R version 4.0.0.
RESULTS
Study Population
Among the 69 884 recipients included in our study, 64 849 received transplants in the prepandemic era and 5035 in the pandemic era. Compared to the prepandemic era, the pandemic era included a greater proportion of deceased-donor transplants (82.2%, pandemic era vs. 68.9%, prepandemic era), because many living donor kidney transplants were postponed or cancelled in the pandemic era.23 We also observed higher proportions of immunologically sensitized recipients (panel reactive antibody≥90; 34.6% vs. 20.6%). Other characteristics were relatively similar between the eras (Table 1).
Table 1.
Population characteristics
| Clinical factors | Pre-COVID (1/1/2017 - 3/12/2020; n=64,849) | Post-COVID (3/13/2020 - 7/31/2020; n=5,035) |
|---|---|---|
| Recipient factors | ||
| Age, y | 53 (41, 63) | 53 (41, 63) |
| Female sex | 39.5% | 38.0% |
| Race | ||
| White | 45.2% | 41.6% |
| African American | 27.0% | 29.4% |
| Hispanic/Latino | 18.6% | 19.5% |
| Other/multiracial | 9.2% | 9.5% |
| Preemptive transplant | 18.9% | 15.7% |
| Time on dialysis, y | 2.4 (0.4, 5.1) | 2.8 (0.7, 5.2) |
| Primary cause of ESRD | ||
| Glumerulonephritis | 27.0% | 25.6% |
| Diabetes | 29.9% | 32.4% |
| Hypertension | 23.0% | 23.9% |
| Others | 20.0% | 18.2% |
| Panel Reactive Antigen | ||
| 0-9 | 54.3% | 50.0% |
| 10-79 | 20.1% | 11.5% |
| 80-89 | 5.0% | 3.8% |
| 90-100 | 20.6% | 34.6% |
| Body mass index, kg/m2 | 27.6 (23.9, 31.8) | 28.1 (24.4, 32.2) |
| Re-transplant | 12.7% | 11.7% |
| Number of HLA-A mismatches | ||
| 0 | 13.8% | 12.7% |
| 1 | 42.6% | 40.9% |
| 2 | 43.6% | 46.4% |
| Number of HLA-B mismatches | ||
| 0 | 8.7% | 7.4% |
| 1 | 30.8% | 27.9% |
| 2 | 60.5% | 64.8% |
| Number of HLA-DR mismatches | ||
| 0 | 17.2% | 16.6% |
| 1 | 48.8% | 48.7% |
| 2 | 34.0% | 34.7% |
| Donor factors | ||
| Age, y | 40 (29, 52) | 39 (29, 50) |
| Female sex | 46.3% | 40.9% |
| Race | ||
| White | 68.6% | 67.1% |
| African American | 12.0% | 13.0% |
| Hispanic/Latino | 14.7% | 15.0% |
| Other/multiracial | 4.6% | 4.8% |
| Type | ||
| Deceased | 68.9% | 82.2% |
| Living | 31.1% | 17.8% |
| Among deceased donors | (n=44,709) | (n=4,139) |
| Cold ischemic time, h | 17.1 (11.7, 22.7) | 16.8 (11.3, 22.3) |
| Donation after circulatory death | 24.1% | 25.1% |
| Donor death due to stroke | 23.0% | 20.4% |
| Pulsatile reperfusion | 42.5% | 44.3% |
| Terminal serum creatinine, mg/dl | 0.9 (0.7, 1.4) | 1.0 (0.7, 1.4) |
ESRD, end-stage renal disease; and HLA, human leukocyte antigen. Numbers indicate median (interquartile range) for continuous variables and percent for categorical variables.
Changes in the use of immunosuppressive agents
The crude percentage of the recipients with a lymphocyte-depleting agent decreased from 73.2% in the prepandemic era to 67.8% in the pandemic era (deceased donor kidney transplant recipients, from 76.2% to 69.9%; and living donor kidney transplant recipients, from 66.5% to 57.7%). Examining individual agents, the use of anti-thymocyte globulin decreased from 54.7% to 54.0% and alemtuzumab from 16.5% to 11.9%, but basiliximab increased from 16.8% to 20.5% and no induction from 6.7% to 7.7%.
When comparing induction agent use in the pandemic era with what would have been expected in the prepandemic area, the use of lymphocyte-depleting agents was significantly lower (Table 2). Before adjustment, the odds of using a lymphocyte-depleting agent (vs. basiliximab or no induction) was 23% lower in the pandemic era compared to the prepandemic era (Model 1; OR=0.720.770.82). The decrease was more pronounced after adjusting for various clinical factors (Model 2; aOR=0.650.700.74), and even more so after accounting for center-level variations (Model 3; aOR=0.400.530.69). Our sensitivity analysis showed similar results as Model 3 did (Model 4; aOR=0.400.520.69), supporting that the small number of recipients at some centers in the pandemic era did not bias our findings. Based on Model 3, the majority of the recipients in the pandemic era would have had a higher probability of receiving a lymphocyte-depleting agent had they received transplants in the prepandemic era (Figure 1a). Across all pandemic era recipients, 74.5% would have received a lymphocyte-depleting agent (vs. 67.8% observed) had they received transplants in the prepandemic era. Additionally, among those who received anti-thymocyte globulin, the average duration of administration was statistically significantly shorter during the pandemic era (3.31 versus 3.17 days in the prepandemic and pandemic eras, respectively; p<0.001).
Table 2.
Changes in the use of lymphocyte-depleting agents and maintenance steroids during the COVID-19 pandemic
| Agent | Crude percentage (Unadjusted) | Odds ratio (pandemic era vs. prepandemic era; adjusted as below) | ||||
|---|---|---|---|---|---|---|
| Prepandemic | Pandemic | Model 1 (unadjusted) | Model 2 (+ patient factors) | Model 3 (+ center-level variation) | Model 4 (sensitivity analysis) | |
| Lymphocyte-depleting agents | 73.2% | 67.8% | 0.720.770.82 | 0.650.700.74 | 0.400.530.69 | 0.400.520.69 |
| Maintenance steroids | 69.1% | 69.3% | 0.951.011.08 | 0.850.900.97 | 0.901.362.07 | 0.911.462.35 |
Model 1 was unadjusted. Model 2 included various recipient factors (age, race, sex, previous history of transplant, body mass index, diabetes, hypertension, primary cause of end-stage renal disease, time on dialysis, serum albumin, previous history of malignancy, previous history of peripheral vascular diseases, primary insurance, hepatitis C virus, and cytomegalovirus), donor factors (deceased vs. living, age, race, sex, body mass index, serum creatinine, hepatitis C virus, cytomegalovirus; for deceased donors – donation after circulatory death, diabetes, hypertension, stroke as the cause of donor death, pulsatile perfusion, and cold ischemic time; and for living donors – biologically related donor), and HLA-A/B/DR matching. Model 3 (main analysis) also adjusted for center-level variation by including random intercepts and random slopes. Model 4 was a sensitivity analysis restricted to 172 (72.3%) transplant centers that performed more than 3 recipients during the pandemic era.
Figure 1. Estimated probability of using lymphocyte-depleting agents and maintenance steroids in the prepandemic versus pandemic era among the pandemic era recipients.
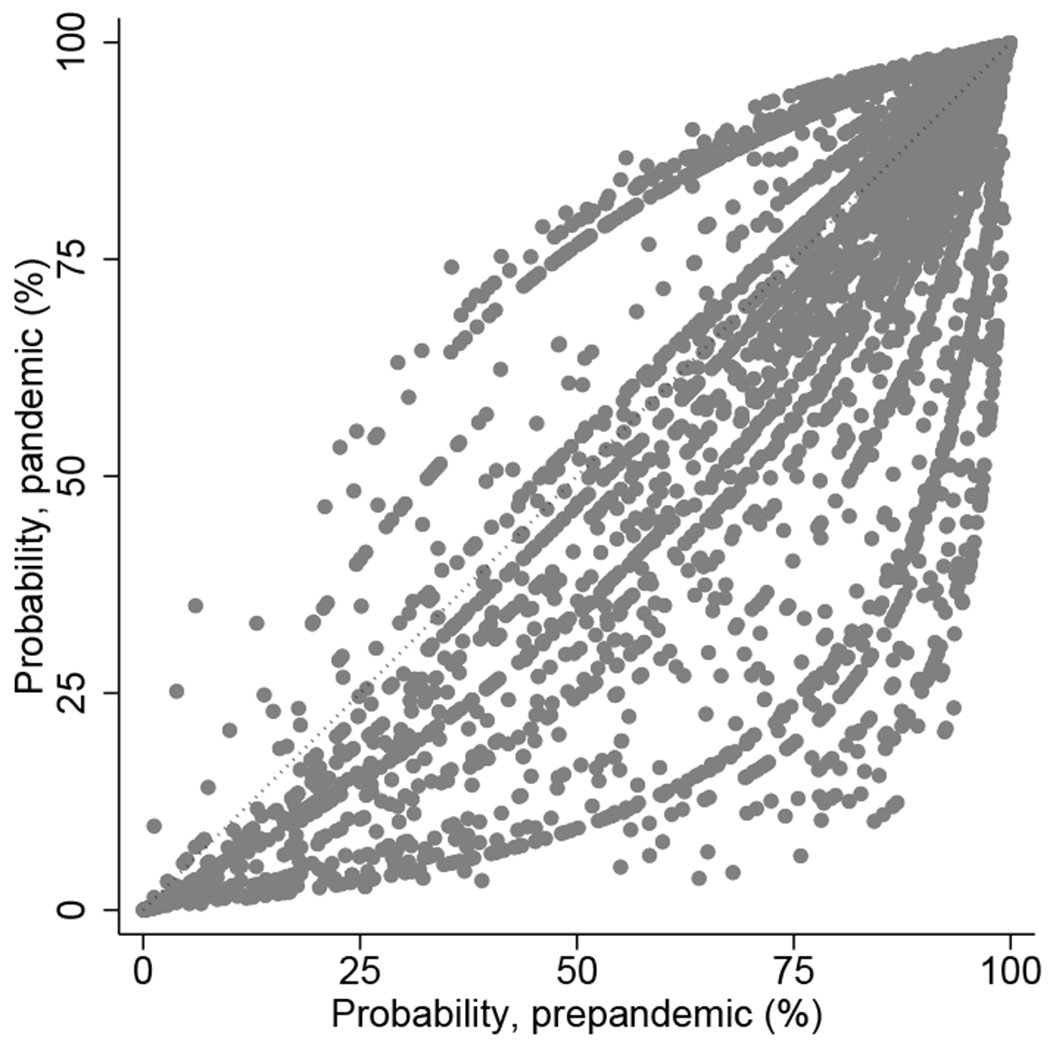
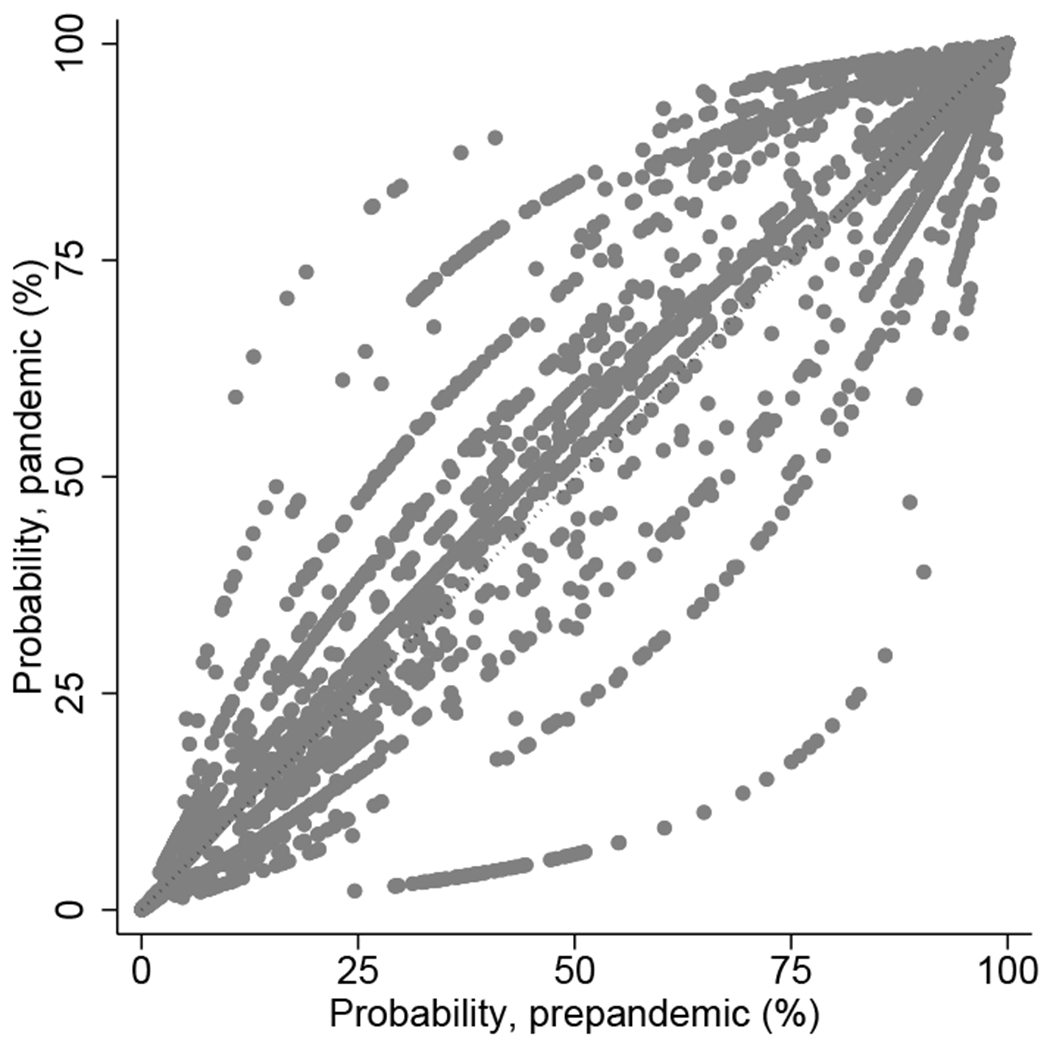
(a) Lymphocyte-depleting agents (vs. nondepleting agent or no induction). (b) Maintenance steroids (vs. early steroid withdrawal).
On the other hand, the percentage of the recipients discharged from the hospital on maintenance steroids remained similar (overall, from 69.1% to 69.3%; deceased donor kidney transplant recipients, from 71.3% to 70.6%; and living donor kidney transplant recipients, from 64.2% to 63.6%). We found no significant change in the use of maintenance steroids between the prepandemic and pandemic eras from any of our models (Table 2). Based on Model 3, the probability of receiving maintenance steroids would have been overall similar had the transplants occurred in the prepandemic era (Figure 1b). Across all pandemic era recipients, 67.8% would have received maintenance steroids (vs. 69.3% observed) had they received transplants in the prepandemic era.
Subgroup analyses
When stratified by state-level COVID-19 incidence, donor type (living vs. deceased), and recipient age, we found no notable difference across the subgroups (Figure 2).
Figure 2. Changes in the use of lymphocyte-depleting agents and maintenance steroids during the COVID-19 pandemic; subgroup analyses.
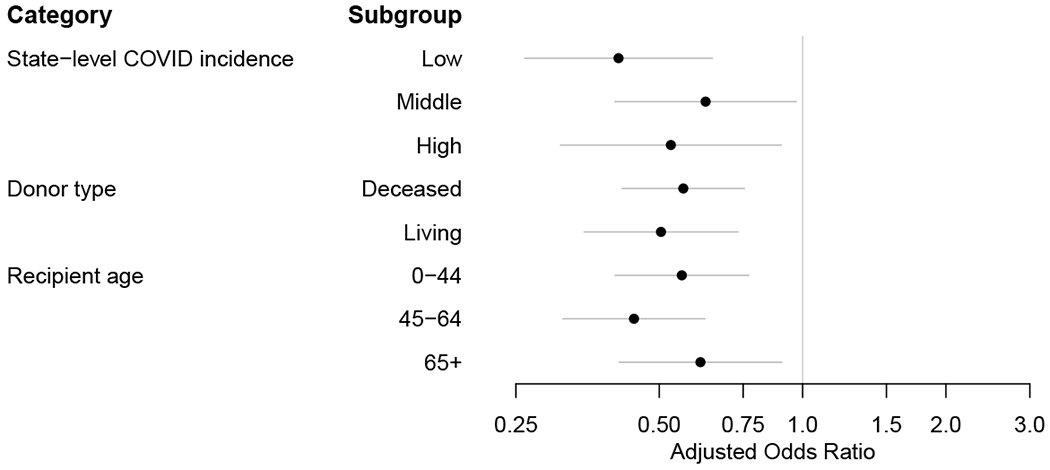
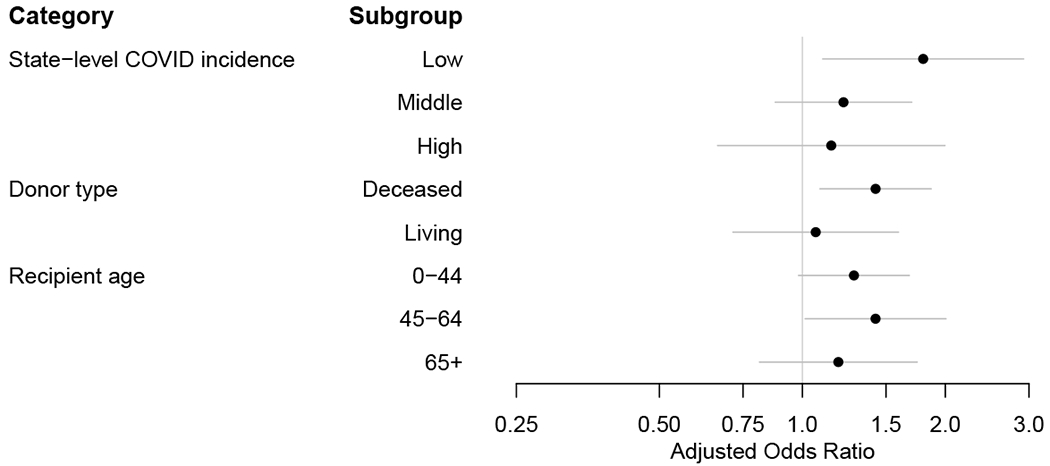
(a) Lymphocyte-depleting agents (vs. nondepleting agent or no induction). (b) Maintenance steroids (vs. early steroid withdrawal).
Early posttransplant outcomes
Among the pandemic era recipients, 43 developed acute rejection during admission, 1,102 developed delayed graft function, 16 developed graft failure, and 39 died. Lymphocyte-depleting agents were associated with substantially lower odds of acute rejection during admission in the pandemic era (aOR=0.110.230.47), similar to the association in the prepandemic era (aOR=0.270.390.58; interaction p=0.2). However, we found no evidence that lymphocyte-depleting agents were associated with increased deaths during the pandemic era (aHR=0.130.471.66). This association was not statistically significantly different from that in the prepandemic era (interaction p=0.3). We found no statistically significant association of lymphocyte-depleting agents with delayed graft function or graft failure (Table 3). Additionally, we found no significant association of maintenance steroid with graft failure or death.
Table 3.
Association of lymphocyte-depleting agents and maintenance steroids with early kidney transplant outcomes in the prepandemic and pandemic eras
| Exposure | Outcome | aOR/aHR |
Interaction p | |
|---|---|---|---|---|
| Prepandemic era | Pandemic era | |||
| Lymphocyte-depleting agent (vs. nondepleting agent or no induction) | Rejection, during admission | 0.270.390.58 | 0.110.230.47 | 0.2 |
| Delayed graft function | 0.800.951.12 | 0.691.121.80 | 0.5 | |
| Graft failure, death-censored | 0.710.911.16 | 0.050.221.07 | 0.1 | |
| Death | 0.820.961.14 | 0.130.471.66 | 0.3 | |
| Maintenance steroid (vs. early steroid withdrawal) | Graft failure, death-censored | 0.710.931.23 | 0.230.722.29 | 0.7 |
| Death | 0.941.231.60 | 0.280.752.04 | 0.4 | |
aOR, adjusted odds ratio (for rejection and delayed graft function); and aHR, adjusted hazard ratio (for graft failure and death). Interaction p<0.05 indicates statistically significant difference in aOR/aHR between the prepandemic and pandemic eras. Rejection during admission and delayed graft function may occur before the selection of maintenance steroid versus early steroid withdrawal, and therefore were not studied as outcomes for maintenance steroid.
DISCUSSION
In this national study of immunosuppression regimens during the first 5 months of the COVID-19 pandemic, we found a substantial decrease in the odds of using lymphocyte-depleting induction (aOR=0.400.530.69), with no clear change in use of maintenance steroids (aOR=0.901.362.07). Similar trends were found across all subgroups of state-level COVID-19 incidence, donor type, and recipient age. However, in the pandemic era, lymphocyte-depleting agents was associated with decreased acute rejection during admission (aOR=0.110.230.47), but not with excess mortality (aHR=0.130.471.66). Our findings do not support that the shift from lymphocyte-depleting agents to basiliximab or no induction was a safe and effective strategy to counterbalance the novel infectious risk during the pandemic.
Since the COVID-19 pandemic is an unprecedented event that renders a unique challenge, there is little scientific evidence to inform if and how the current clinical practice should be adjusted to address this challenge. Generally, lymphocyte-depleting agents are known to achieve a higher level of immunosuppression, which might theoretically increase the risk of infections more than basiliximab or no induction.5,24 However, clinical evidence on the association of lymphocyte-depleting agents versus basiliximab or no induction with general infection risk is inconclusive.1,25,26 Even in higher risk recipient subgroups like those with HIV, the advantages and disadvantages of lymphocyte-depleting induction remains controversial.27,28 In this light, reducing induction during the pandemic might increase risks of rejection without any infectious advantages, or might outweigh any risks of rejection with protection from novel coronavirus infection. This hypothesis is supported by the results from our outcomes analyses, which showed a decrease in acute rejection but no significant difference in mortality associated with lymphocyte-depleting agents among the pandemic era recipients.
Our study focused on choice of induction agent and early use of steroids at transplant discharge. We did not assess immunosuppression management in patients who acquired COVID-19 infection after transplant, including antimetabolite and calcineurin inhibitor use, which are topics of active debate.29,30 Incident transplant recipients should be free of COVID-19 at the time of organ implantation based on contemporary screening practices. Development of COVID-19 early after kidney transplant such as during the transplant hospitalization could impact use of steroids and other agents (as reactive rather than preventative management) but to date such early cases have been rare.
Our study has several limitations that merit exploration. First, due to the observational design of our study, we cannot determine the true reasons for the observed decrease in the use of lymphocyte-depleting agents. Second, we were not able to examine the dose of the immunosuppressive agents, as the SRTR datasets do not include dose data. It is possible that some transplant providers reduced the dose of immunosuppressive agents, as opposed to switching to milder options, so any findings of “no change” need to be considered in that light. Third, because of data reporting reliability, we limited our study to only immunosuppression decisions during the transplant hospitalization and immediate discharge, so we could not evaluate changes in maintenance immunosuppression (other than early steroid withdrawal). Fourth, some of the transplant centers might have been underrepresented in the pandemic era if the centers were functioning at reduced capacity, completely shut down, or late reporting data (especially immunosuppression data) to OPTN/UNOS. Nonetheless, our multilevel modeling (Model 3) accounts for this issue, and our sensitivity analyses (Model 4) found similar results even after excluding centers with little pandemic-era data. Finally, our analyses on posttransplant outcomes are based on relatively short follow-up periods and only include endpoints reported to UNOS/OPTN by July 31, 2020.
To summarize, we observed a decrease in the use of lymphocyte-depleting agents during the first several weeks of the COVID-19 pandemic compared to the 3-year period preceding the pandemic. Similar trends were found across all subgroups of state-level COVID-19 incidence, donor type, and recipient age. However, lymphocyte-depleting agents were associated with decreases in rejections but with no significant difference in mortality in the pandemic era, casting doubt on whether the shift in induction immunosuppression was a safe and effective approach to address the novel infectious risk during the pandemic.
Acknowledgments
The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government. The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
FUNDING
This research was made possible with generous support of the Ben-Dov family. This work was supported by grant number R01DK120518 (McAdams-DeMarco), K01DK101677 (Massie) and K24DK101828 (Segev) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and T32AI007291-27 (Werbel) from the National Institute of Allergy and Infectious Diseases (NIAID).
ABBREVIATIONS
- COVID-19
Coronavirus disease 2019
- HRSA
Health Resources and Services Administration
- IPTW
inverse probability of treatment weights
- OPTN
Organ Procurement and Transplantation Network
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
DISCLOSURE
DLS receives speaking honoraria from Sanofi, Novartis, and CSL Behring. All other authors declare no conflicts of interest.
References
- 1.Brennan DC, Daller JA, Lake KD, et al. Rabbit Antithymocyte Globulin versus Basiliximab in Renal Transplantation. N Engl J Med. 2006;355(19):1967–1977. [DOI] [PubMed] [Google Scholar]
- 2.Webster AC, Woodroffe RC, Taylor RS, et al. Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: meta-analysis and meta-regression of randomised trial data. BMJ. 2005;331(7520):810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matas AJ, Kandaswamy R, Humar A, et al. Long-term Immunosuppression, Without Maintenance Prednisone, After Kidney Transplantation: Ann Surg. 2004;240(3):510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodle ES, First MR, Pirsch J, et al. A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann Surg. 2008;248(4):564–577. [DOI] [PubMed] [Google Scholar]
- 5.Fishman JA. Infection in Solid-Organ Transplant Recipients. N Engl J Med. 2007;357(25):2601–2614. [DOI] [PubMed] [Google Scholar]
- 6.Snyder JJ, Israni AK, Peng Y, et al. Rates of first infection following kidney transplant in the United States. Kidney Int. 2009;75(3):317–326. [DOI] [PubMed] [Google Scholar]
- 7.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2009;9 Suppl 3:S1–155. [DOI] [PubMed] [Google Scholar]
- 8.Wavamunno MD, Chapman JR. Individualization of immunosuppression: concepts and rationale. Curr Opin Organ Transplant. 2008;13(6):604–608. [DOI] [PubMed] [Google Scholar]
- 9.Hellemans R, Bosmans J- L, Abramowicz D. Induction Therapy for Kidney Transplant Recipients: Do We Still Need Anti-IL2 Receptor Monoclonal Antibodies? Am J Transplant. 2017;17(1):22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleming JN, Taber DJ, Pilch NA, et al. Yes, We Still Need IL-2 Receptor Antagonists. Am J Transplant. 2016;16(11):3308–3309. [DOI] [PubMed] [Google Scholar]
- 11.Matas AJ, Gaston RS. Moving Beyond Minimization Trials in Kidney Transplantation. J Am Soc Nephrol. 2015;26(12):2898–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pereira MR, Mohan S, Cohen DJ, et al. COVID‐19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020;20(7):1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coates PT, Wong G, Drueke T, et al. Early experience with COVID-19 in kidney transplantation. Kidney Int. 2020;97(6):1074–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernández‐Ruiz M, Andrés A, Loinaz C, et al. COVID‐19 in solid organ transplant recipients: A single‐center case series from Spain. Am J Transplant. 2020;20(7):1849–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung K, Wu JT, Liu D, et al. First-wave COVID-19 transmissibility and severity in China outside Hubei after control measures, and second-wave scenario planning: a modelling impact assessment. The Lancet. 2020;395(10233):1382–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kissler SM, Tedijanto C, Goldstein E, et al. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368(6493):860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massie AB, Kuricka LM, Segev DL. Big Data in Organ Transplantation: Registries and Administrative Claims: Big Data in Organ Transplantation. Am J Transplant. 2014;14(8):1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haller MC, Royuela A, Nagler EV, et al. Steroid avoidance or withdrawal for kidney transplant recipients. Cochrane Database Syst Rev. 2016;(8):CD005632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bae S, Garonzik Wang JM, Massie AB, et al. Early Steroid Withdrawal in Deceased-Donor Kidney Transplant Recipients with Delayed Graft Function. J Am Soc Nephrol JASN. 2020;31(1):175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trump DJ. Proclamation on Declaring a National Emergency Concerning the Novel Coronavirus Disease (COVID-19) Outbreak. Accessed on May 24, 2020 Available at https://www.whitehouse.gov/presidential-actions/proclamation-declaring-national-emergency-concerning-novel-coronavirus-disease-covid-19-outbreak/.
- 21.Novel Coronavirus (COVID-19) Cases, provided by JHU CSSE. GitHub. Accessed June 10, 2020 Available at: https://github.com/CSSEGISandData/COVID-19.
- 22.Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostat Oxf Engl. 2009;10(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar D, Manuel O, Natori Y, et al. COVID-19: A global transplant perspective on successfully navigating a pandemic. Am J Transplant. 2020;20(7):1773–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mueller NJ. New immunosuppressive strategies and the risk of infection. Transpl Infect Dis. 2008;10(6):379–384. [DOI] [PubMed] [Google Scholar]
- 25.Kim JM, Jang HR, Kwon CHD, et al. Rabbit Antithymocyte Globulin Compared with Basiliximab in Kidney Transplantation: A Single-Center Study. Transplant Proc. 2012;44(1):167–170. [DOI] [PubMed] [Google Scholar]
- 26.Jorge S, Guerra J, Silva S, et al. Induction Immunosuppressive Therapy in Renal Transplantation: Does Basiliximab Make the Difference? Transplant Proc. 2008;40(3):693–696. [DOI] [PubMed] [Google Scholar]
- 27.Kucirka LM, Durand CM, Bae S, et al. Induction Immunosuppression and Clinical Outcomes in Kidney Transplant Recipients Infected With Human Immunodeficiency Virus. Am J Transplant. 2016;16(8):2368–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stock PG, Barin B, Murphy B, et al. Outcomes of Kidney Transplantation in HIV-Infected Recipients. N Engl J Med. 2010;363(21):2004–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maggiore U, Abramowicz D, Crespo M, et al. How should I manage immunosuppression in a kidney transplant patient with COVID-19? An ERA-EDTA DESCARTES expert opinion. Nephrol Dial Transplant. 2020;35(6):899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Society of Transplantation. 2019-nCoV (Coronavirus): FAQs for Organ Transplantation. American Society of Transplantation. [Google Scholar]


