Figure 4. Fusion of B domain to PF-A significantly increases ANS fluorescence.
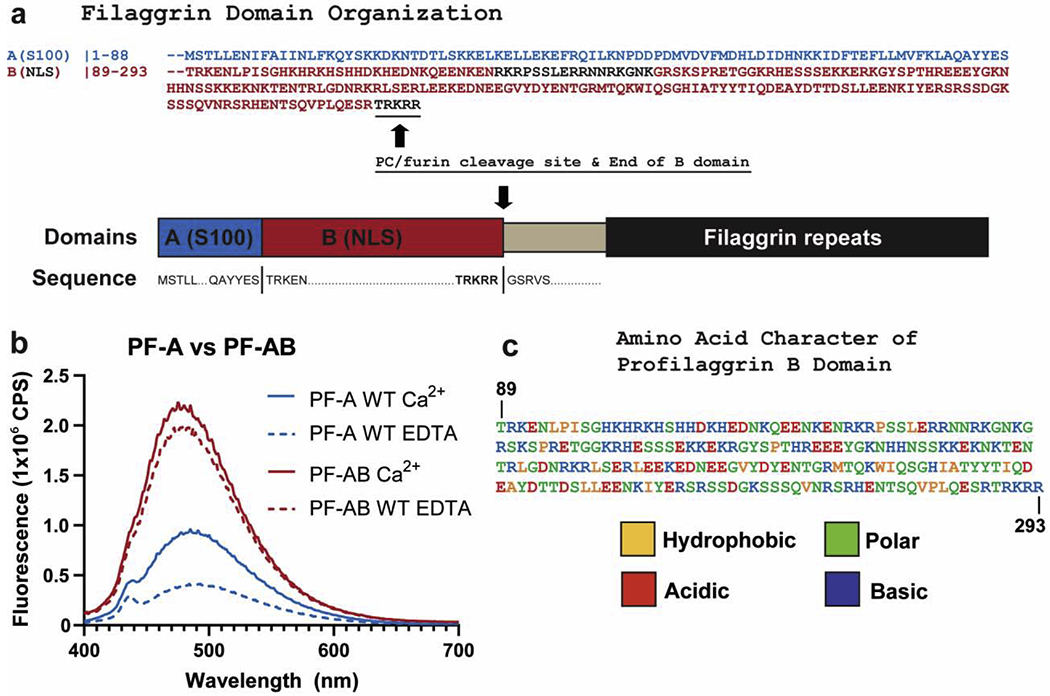
a) Schematic of the domains of the N-terminus of human profilaggrin with amino acid sequences listed for the beginning and termination of each domain. The B domain ends at the PC/furin cleavage site “RTRKRR.” b) Comparison of ANS fluorescence between wild-type PF-A and PF-AB in the presence of Ca2+ or EDTA. Wild-type PF-A undergoes the expected Ca2+-dependent conformational opening and increase in ANS fluorescence (blue). PF-AB, however, demonstrates a markedly robust binding and fluorescence of ANS (red), which is slightly stronger in the presence of Ca2+. c) PF-B amino acid sequence colored to reflect the hydrophobic, polar, acidic, and basic character of this domain as determined by ProtParam analysis. Abbreviations: NLS, nuclear localization signal; NTR, N-terminal truncated repeat.
