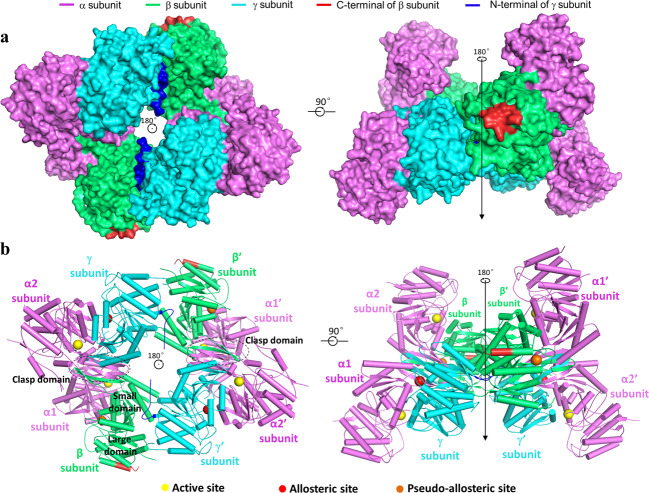
Fig. 1. Overall structure of the apo β-mutant HsIDH3.
a Surface and b cartoon presentations of the apo β-mutant HsIDH3 in two different orientations. Left: view along the crystallographic 2-fold axis of the heterooctamer of HsIDH3. Right: view in perpendicular to the crystallographic 2-fold axis of the heterooctamer of HsIDH3. The color coding of the α, β, and γ subunits is shown above. The N-terminal regions of the γ subunits and the C-terminal substituted regions of the β subunits are colored in blue and red, respectively. The clasp domains of the αβ and αγ heterodimers are indicated with dashed ovals. The active sites, the allosteric sites, and the pseudo allosteric sites are indicated with yellow, red, and orange spheres, respectively. Residues and structure elements of the α and β subunits of the αβ heterodimer and the α and γ subunits of the αγ heterodimer are superscripted by “A1” and “B”, and “A2” and “G”, respectively.