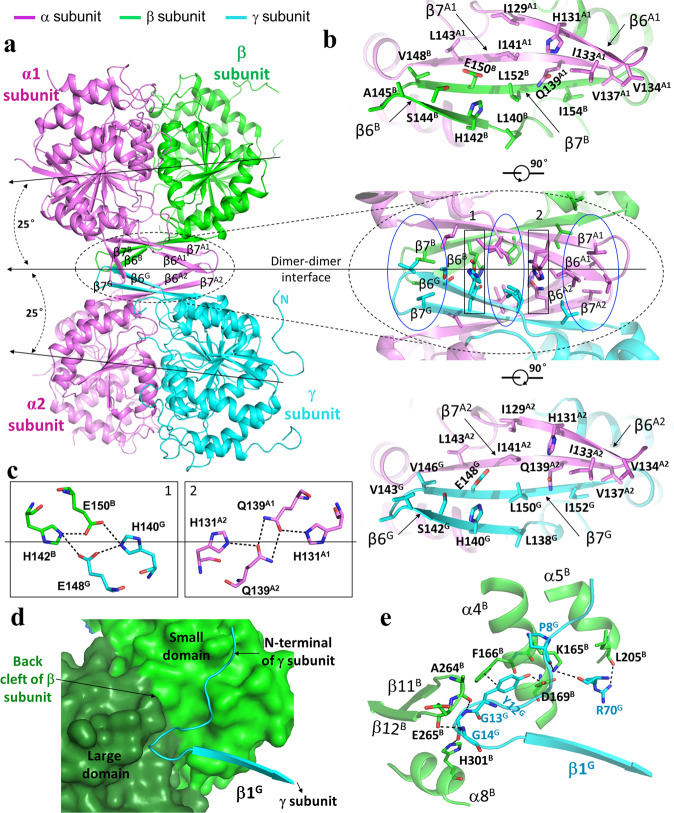
Fig. 2. Interactions at the heterodimer–heterodimer and the heterotetramer–heterotetramer interfaces in the apo β-mutant HsIDH3 structure.
a The α2βγ heterotetramer is assembled by the αβ and αγ heterodimers via their clasp domains. The color coding of the α, β, and γ subunits is the same as in Fig. 1. The pseudo 2-fold axis along the heterodimer–heterodimer interface, and the coplane axes of the αβ and αγ heterodimers are indicated. b Structure of the heterodimer–heterodimer interface. Middle panel: interactions at the interface consist of largely hydrophobic residues (marked by blue ovals) and a few hydrophilic residues (marked by black rectangles). Upper panel: interactions between the α and β subunits at the interface. Lower panel: interactions between the α and γ subunits at the interface. c Hydrogen-bonding interactions between the β and γ subunits (left panel) and between the two α subunits (right panel). d A surface diagram showing that the N-terminal region of the γ subunit (in cyan ribbon) of one heterotetramer lies in a shallow cleft (the “back cleft”) formed by the small and large domains of the β subunit (in green and dark green surface, respectively) of the other heterotetramer. e Interactions between the γ subunit of one heterotetramer (in cyan) and the β subunit of the other heterotetramer (in green). The hydrogen-bonding interactions are indicated with dashed lines.