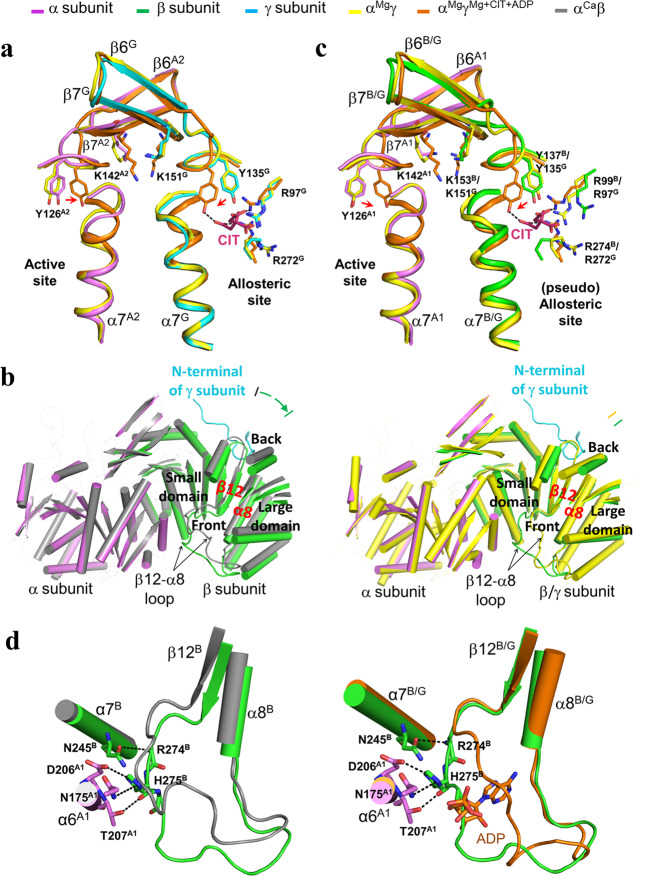
Fig. 3. Structural comparisons of the αβ and αγ heterodimers in the apo β-mutant HsIDH3 and in the isolated forms.
a Comparison of the αγ heterodimer in the heterooctamer of HsIDH3 and in the isolated forms at the heterodimer interface. The color coding of the subunits and structures is shown above. The key residues at the active site (Tyr126A2), the allosteric site (Arg97G, Tyr135G, and Arg272G), and the heterodimer interface (Lys151G and Lys142A2) assume similar conformations as those in the inactive αMgγ structure (PDB code: 5GRH) rather than those in the active αMgγMg+CIT+ADP structure (PDB code: 5GRF). b Comparison of the overall conformation of the αβ heterodimer in the heterooctamer of HsIDH3 with that of the isolated αCaβ heterodimer (PDB code: 6KDE) (in gray, left panel) and αMgγ heterodimer (in yellow, right panel). The αβ heterodimer assumes an open overall conformation similar to that of the isolated αMgγ heterodimer rather than the compact conformation of the isolated αCaβ heterodimer. For clarity, only the α helices and β strands are shown, and the loops are omitted except the β12-β8 loops of the β and γ subunits. The N-terminal of the γ subunit from another heterotetramer is also shown. c Comparison of the αβ heterodimer in the heterooctamer of HsIDH3 with the isolated αMgγ heterodimer and αMgγMg+CIT+ADP heterodimer. The key residues at the active site (Tyr126A1), the pseudo allosteric site (Arg99B, Tyr137B, and Arg274B), and the heterodimer interface (Lys153B and Lys142A1) assume similar conformations as those in the inactive αMgγ structure rather than those in the active αMgγMg+CIT+ADP structure. d The β12B-β8B loop of the β subunit in the heterooctamer of HsIDH3 exhibits some conformational differences from that in the isolated αCaβ heterodimer but still occupies the ADP-binding site in the αMgγMg+CIT+ADP structure. The hydrogen-bonding interactions of the β12B-β8B loop with the α7B and α6A1 helices are indicated with dashed lines.