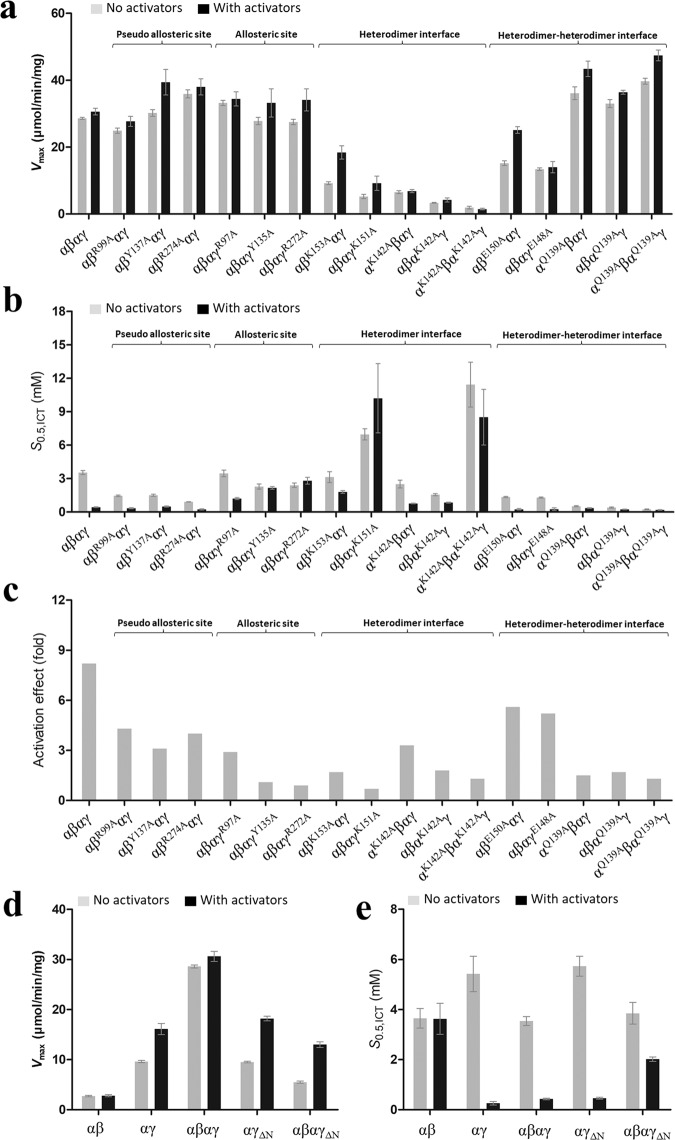
Fig. 4. Effects of the mutations on the activity and allosteric activation of HsIDH3.
a Graph presentations of the Vmax values, b the S0.5,ICT values, and c the activation effects of wild-type HsIDH3 and the HsIDH3 mutants containing mutations of key residues at the allosteric site, the pseudo allosteric site, the heterodimer interfaces, and the heterodimer–heterodimer interface in the absence or presence of CIT and ADP. The activation effect is defined as the ratio of the S0.5,ICT in the absence and presence of the activators. The detailed kinetic parameters are listed in Table 2. d Graph presentations of the Vmax values and e the S0.5,ICT values of wild-type αβ and αγ heterodimers, wild-type α2βγ heterooctamer, the mutant αγΔN heterodimer, and the mutant α2βγΔN heterotetramer in the absence and presence of CIT and ADP. The detailed kinetic parameters are listed in Table 2.