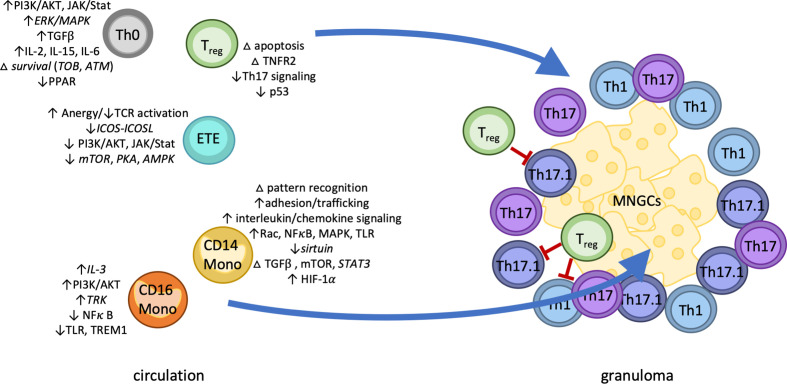
Figure 4.
The four pillars of sarcoidosis model. Classical monocytes upregulated distinct markers of activation including adhesion molecules, pattern recognition receptors, and chemokine receptors, as well as enrichment of immunoregulatory pathways HMGB1, mTOR, and ephrin receptor signaling. Predictive modeling implicated TGFβ and mTOR signaling as drivers of persistent classical monocyte activation. In contrast, CD16 monocytes displayed both up- and down-regulation of a small number of inflammatory pathways. Sarcoidosis T cells subsets also displayed patterns of dysregulation. CD4 naïve T cells were enriched for markers of apoptosis and Th17/Treg differentiation, while effector T cells showed enrichment of anergy-related pathways. Differentially expressed genes in regulatory T cells suggested dysfunctional p53, cell death, and TNFR2 signaling. We therefore hypothesize sarcoidosis pathology is marked by four distinct cell-specific effects: 1) persistent hyperactivation of innate immunity via classical monocytes 2) in combination with CD4 naïve T cell activation and 3) exacerbated by regulatory T cell dysfunction and potentially 4) self-reactive effector T cells potentially suppressed by anergy. Abbreviations: Th0, CD4 naïve T cell; ETE, early T effector; Treg, regulatory T cells; MNGCs, multi-nucleated giant cells; Th1/17/17.1, differentiated helper T cells; LN, lymph node; CD14 mono, classical (CD14+) monocytes; CD16 mono, non-classical (CD16+) monocytes. Italics indicate novel discovery of basal gene- or protein-level enrichment of the indicated pathway in the indicated cell type in sarcoidosis.