Fig. 4.
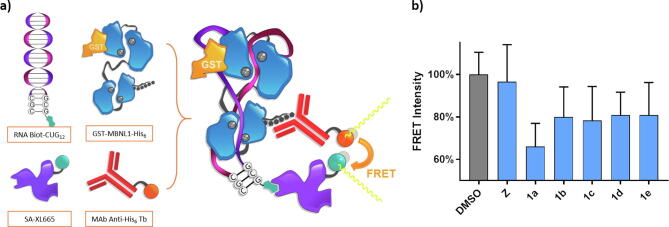
a) Graphical representation of AID2675 FRET test molecular basis and its principal components: 5′-biotinylated RNA oligo Biot-(CUG)12 and the recombinant GST-MBNL1-His6 form a stable complex. FRET donor anti-His6-Terbium cryptate and FRET acceptor streptavidin-conjugated XL665 bind to His tag and Biotin, respectively. While no active compound is present in the reaction media, 340 nm beam light hits Tb conjugate and this is close enough to transfer the energy to XL665 conjugate emitting at 665 nm. If a compound can free the protein no FRET occurs, and Tb fluorophore emits at 545 nm. b) Normalized FRET intensity measured as a relationship between the fluorescence intensity of the signals of 545 and 665 nm (average of three measures). Final concentrations of studied candidates were set at 0.1 µM and 20 nM of protein-RNA complex. Z is included for comparison.