Table 1.
Mushroom tyrosinase inhibition of the synthesized (Z)-5-(substituted benzylidene)-3-phenyl-2-thioxooxazolidin-4-one derivatives 1a–1p and kojic acid.
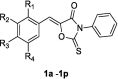 | |||||
|---|---|---|---|---|---|
| Compound | R1 | R2 | R3 | R4 | Tyrosinase inhibition (%)a |
| 1a | H | H | OH | H | 10.25 ± 2.36 |
| 1b | H | OH | OH | H | 30.57 ± 1.77 |
| 1c | OH | H | OH | H | 78.05 ± 4.03 |
| 1d | H | OMe | OH | H | NIb |
| 1e | H | OEt | OH | H | NI |
| 1f | H | OH | OMe | H | 13.39 ± 9.67 |
| 1g | H | H | OMe | H | NI |
| 1h | OMe | H | OMe | H | 4.09 ± 3.66 |
| 1i | H | OMe | OMe | H | 7.47 ± 5.52 |
| 1j | OH | H | H | H | 71.12 ± 0.71 |
| 1k | H | OMe | OMe | OMe | NI |
| 1l | H | OMe | OH | OMe | 10.22 ± 4.82 |
| 1m | H | t-Bu | OH | t-Bu | 8.53 ± 2.25 |
| 1n | H | Br | OH | H | 24.96 ± 4.72 |
| 1o | H | Br | OH | Br | NI |
| 1p | F | H | F | H | NI |
| Kojic acid | 58.09 ± 5.82 | ||||
aTyrosinase inhibitions of the synthesized compounds and kojic acid were evaluated at 25 μM using L-tyrosine as a substrate. bNI: no inhibition. Results are expressed as means ± SEMs.
