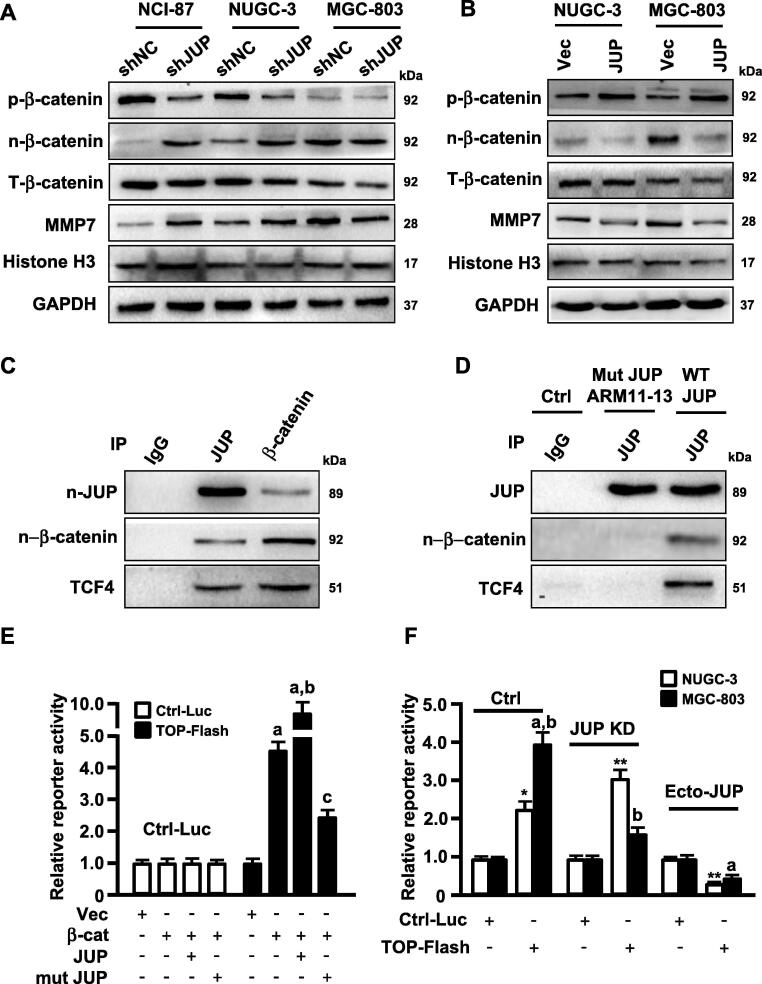
Fig. 6.
JUP mediates MMP7 expression via the interaction of JUP/β -catenin/TCF4. (A, B) Levels of phosphorylated β-catenin (p-β-catenin), nuclear β-catenin (n-β-catenin), total β-catenin (T-β-catenin) and MMP7 were determined by Western blot in the JUP knocked-down (A) and overexpressing (B) gastric cancer cells and its control cells. Histone H3 and GAPDH are the loading control. (C, D) Whole-cell lysates from WT MGC-803 (C) and JUP ARM11-13 mutant MGC-803 cell (D) were immunoprecipitated with anti-JUP and anti-β-catenin antibodies. Western blot showed the interaction of JUP, β-catenin and TCF4. IgG was used as a control antibody. (E) 293 T cells were co-transfected with control luciferase reporter or TOP-Flash reporter and indicated constructs, relative reporter activity were measured for TCF4 (a, P < 0.01 vs vector; b, P < 0.01, β -catenin/JUP vs β -catenin alone; c, P < 0.01, β -catenin/mutant JUP vs β -catenin/JUP). (F) NUGC-3 and MGC-803 and their engineered cells (JUP-knocked down cells and ectopic JUP-overexpressed cells) were transfected with control luciferase reporter or TOP-Flash reporter. Endogenous TCF4 transcript activity was detected using luciferase assay (*P < 0.05, **P < 0.01 vs Control reporter; a, P < 0.01, TOP-Flash reporter vs control reporter; b, P < 0.01, MGC-803 vs NUGC-3).