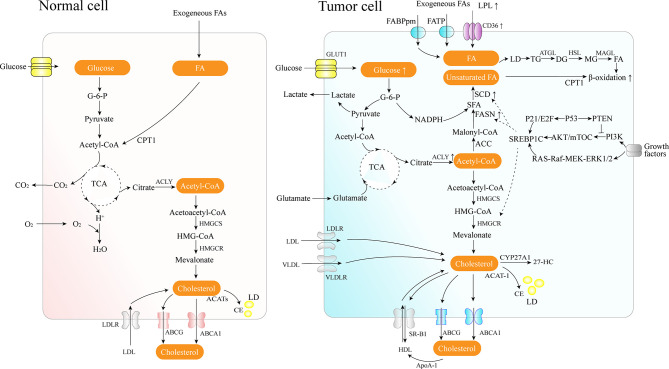
Figure 1.
Lipid metabolism overview in normal and cancer cells. Cancer cells acquire diet-derived FA through LPL, CD36, FATPs, and FABPpm. Glucose is converted to acetyl-CoA by glycolysis and on to citrate through the TCA cycle in the mitochondria. The citrate is transported to the cytoplasm and converted back to acetyl-CoA by citrate lyase, which is used as the carbon source for the growing acyl chains. The pentose phosphate pathway from glycolysis generates NADPH. Cancer cells also develop effective de novo FAS machinery with an increase in the activity of key lipogenic enzymes. The surplus lipids (including excess FAs and cholesterol) in a cell exist in the form of neutral, inert biomolecules in the core of LDs. ATGL catalyzes the initial step of lipolysis, converting TGs to DGs; HSL is primarily responsible for the hydrolysis of DGs to MGs, and MAGL hydrolyzes MGs into FFA and glycerol. CPT1, as an outer mitochondrial membrane enzyme, translocates FA across the mitochondrial membranes and then the degradation of long-chain FAs occurs in the mitochondria. Cholesterol homeostasis involves the interplay between de novo synthesis (mevalonate pathway), uptake of dietary cholesterol, and removal of excess cholesterol from peripheral tissues. 27-HC is the metabolite substrate of cholesterol by CYP27A1 enzymes. SREBP-1 is activated through the PI3K/Akt/mTOR pathway and the Ras/Raf/MEK/ERK signaling pathway.