Abstract
Objectives
The aims of this study are to report the prevalence of delirium on admission to the unit in patients hospitalized with SARS‐CoV‐2 infection, to identify the factors associated with delirium, and to evaluate the association between delirium and in‐hospital mortality.
Design
Multicenter observational cohort study.
Settings
Acute medical units in four Italian hospitals.
Participants
A total of 516 patients (median age 78 years) admitted to the participating centers with SARS‐CoV‐2 infection from February 22 to May 17, 2020.
Measurements
Comprehensive medical assessment with detailed history, physical examinations, functional status, laboratory and imaging procedures. On admission, delirium was determined by the Diagnostic and Statistical Manual of Mental Disorders (5th edition) criteria, 4AT, m‐Richmond Agitation Sedation Scale, or clinical impression depending on the site. The primary outcomes were delirium rates and in‐hospital mortality.
Results
Overall, 73 (14.1%, 95% confidence interval (CI) = 11.0–17.3%) patients presented delirium on admission. Factors significantly associated with delirium were dementia (odds ratio, OR = 4.66, 95% CI = 2.03–10.69), the number of chronic diseases (OR = 1.20, 95% CI = 1.03; 1.40), and chest X‐ray or CT opacity (OR = 3.29, 95% CI = 1.12–9.64 and 3.35, 95% CI = 1.07–10.47, for multiple or bilateral opacities and single opacity vs no opacity, respectively). There were 148 (33.4%) in‐hospital deaths in the no‐delirium group and 43 (58.9%) in the delirium group (P‐value assessed using the Gray test <.001). As assessed by a multivariable Cox model, patients with delirium on admission showed an almost twofold increased hazard ratio for in‐hospital mortality with respect to patients without delirium (hazard ratio = 1.88, 95% CI = 1.25–2.83).
Conclusion
Delirium is prevalent and associated with in‐hospital mortality among older patients hospitalized with SARS‐CoV‐2 infection.
Keywords: COVID‐19, older adults, hospital, mortality, delirium
1. INTRODUCTION
Soon after the start of the severe acute respiratory syndrome 2 (SARS‐CoV‐2) novel coronavirus (COVID‐19) pandemic, it became clear that older adults are among those most at risk of health‐related negative outcomes. Rates of morbidity and mortality associated with COVID‐19 have been found to be much higher in older adults than in all other age groups. 1 , 2 It has also been recognized that atypical presentations of COVID‐19 are more frequent in these segments of the population. 3 , 4
Delirium is an atypical presenting symptom of many acute diseases and a very common condition among older hospital inpatients. 5 , 6 The fact that delirium has negative prognostic implications for older people 6 , 7 suggests that accurate data on its estimated prevalence and outcomes in patients with SARS‐CoV‐2 infection are urgently needed to allow planning of appropriate interventions for people who will be hospitalized with COVID‐19 in the coming months.
To date, delirium prevalence in COVID‐19 varies greatly between studies, with some, published in the early months of the pandemic, reporting high or very high rates, 3 , 8 , 9 , 10 , 11 and other more recent ones finding lower prevalence. 12 , 13 , 14 Furthermore, there are only a few studies that have investigated the risk factors of delirium in patients with COVID‐19 15 , 16 and there are contrasting findings regarding the potential association of delirium with mortality in this context. 3 , 8 , 9 , 13 , 14 , 15 There is therefore a need for further exploration of these issues.
The aims of this study, conducted in a large population of patients admitted to acute medical and rehabilitation wards with SARS‐CoV‐2 infection, are to report the prevalence of delirium on admission to the units, identify the factors associated with delirium occurrence, and evaluate the association between delirium and in‐hospital mortality.
2. METHODS
2.1. Study Population
This observational study was conducted at four different hospitals in northern Italy: one large, urban, tertiary hospital (“San Gerardo,” 650 beds) in Monza, two urban, private hospitals (“Poliambulanza,” 600 beds, and “Sant'Anna,” 300 beds) in Brescia, and one small, private, rehabilitation hospital (Fondazione Teresa Camplani, 120 beds) in Cremona.
Data collection complied fully with European law on personal data protection. Oral informed consent for participation in this study was obtained from patients or proxy respondents on admission, and the study protocol was approved by the Brianza Institutional Review Board.
The study population comprised consecutive patients admitted, between February 22, 2020 and May 17, 2020, to two acute geriatric units (at the San Gerardo and Poliambulanza hospitals, respectively), one acute medical unit (Sant'Anna hospital), and one rehabilitation unit (Fondazione Teresa Camplani hospital) with a positive polymerase chain reaction nasopharyngeal swab test for SARS‐CoV‐2. The patients admitted to the three acute units came from the emergency department, while those admitted to the rehabilitation unit were transferred from the acute wards of the hospital (Cremona). There was no preliminary selection of patients by physicians at the participating centers. Exclusion criteria were aged less than 65 years and/or initial admission to an intensive care unit (ICU). Patients were followed until death, discharge or transfer from the above‐mentioned units. Follow‐up was updated on June 16, 2020 (date inclusive).
2.2. Comprehensive Medical Assessment
All patients underwent by the attending physician a comprehensive medical assessment on unit's admission, which included detailed history and physical examination. Data on all clinical characteristics, including patient demographics (age, sex), smoking habits, functional status, cognitive status, clinical and pharmacological history, chest X‐ray or CT, use of continuous positive airway pressure (CPAP), and laboratory results were collected in a structured data form. However, the tools used for these assessments differed between the centers. In centers 1 (San Gerardo hospital) and 2 (Sant'Anna hospital), functional status was assessed using some items of Katz's Activities of Daily Living (ADL) scale 17 and the Instrumental ADL, 18 while the Barthel Index 19 was used in Centers 3 (Poliambulanza Hospital) and 4 (Fondazione Teresa Camplani Hospital). To combine these different functional status measures, we built an indicator of functional disability. Accordingly, functional disability corresponded to: dependence in bathing or dressing (for Centers 1 and 2) or a Barthel Index score 19 90 or more/100 one month before hospitalization (for Centers 3 and 4). In all four centers, dementia was diagnosed in the presence of a documented history. Comorbidity was measured using the Cumulative Illness Rating Scale (CIRS) 20 in Centers 3 and 4, and using a checklist of chronic diseases in Centers 1 and 2. Diagnosis of nutritional disorders (including both malnutrition and obesity) was based on the attending physicians' clinical judgment.
We also recorded the presence and degree of chest X‐ray or CT infiltrates or opacities and the use of use of continuous positive airway pressure (CPAP) for respiratory support. Furthermore, in all centers, serum levels of albumin (except Center 4), leukocytes and C‐reactive protein (CRP) were recorded.
2.3. Delirium Assessment
Under normal circumstances, patients at centers are routinely assessed for delirium as follows: at Centers 1 and 4, the patients are usually screened by geriatricians with the 4‐AT tool 21 and the diagnosis of delirium is confirmed according to the DSM‐5 criteria. 22 The modified Richmond Agitation‐Sedation Scale (m‐RASS) 23 is used to evaluate the patient's arousal and support the diagnosis of delirium. At Centers 2 and 3, the physicians on site do not routinely employ screening tools for delirium and the diagnosis of the condition is based on their clinical impression. During the COVID‐19 emergency, however, the medical staff has changed in all centers but Center 4, including various specialists which were not trained in delirium evaluation (i.e., thoracic surgeons, dermatologists and gastroenterologists). Consequently, the methods to diagnose delirium have varied from usual practice, depending on the center and the expertise of physicians: at Center 1, when the attending physician was a geriatrician, delirium diagnoses were made using the same procedures as in non‐emergency situations. However, when a geriatrician was not on duty, these diagnoses were based on the physician's clinical impressions. At the other centers, delirium diagnosis was made employing the same procedures as in normal circumstances (i.e., based on the physician's clinical impression at Centers 2 and 3, while based on 4AT screening and DSM‐5 confirmation at Center 4).
Because an altered arousal is highly specific for delirium 24 and the m‐RASS score (i.e., a measure of arousal) was obtained on patient's admission in three centers, we decided to retrospectively re‐classify as delirium all the cases in Center 1 in which the diagnosis was not obtained by a geriatrician and the m‐RASS score was different than 0. At Center 2, the cases of delirium were reclassified using the same latter approach (i.e., a m‐RASS score different than 0). At Center 3, the m‐RASS score was disregarded to reclassify the delirium episodes.
The Supplementary Table S1 summarizes the methods to assess delirium at each center during COVID‐19 emergency.
2.4. Primary and Secondary Outcomes
The primary outcome was the rate of delirium among all patients with SARS‐Cov‐2 infection on admission to the participating centers. The secondary outcome was all‐cause in‐hospital mortality.
2.5. Data Collection
Clinical, demographic, and laboratory data were prospectively collected in an electronic database. At the end of the study period these data and all clinical and nursing notes were reviewed.
Dates of in‐hospital deaths were also recorded.
2.6. Statistical Analyses
Continuous variables were described with median and interquartile range (IQR), and categorical data were expressed as frequency and percentage values. The clinical features of the patients with and without delirium on admission were compared by Mann‐Whitney test and Fisher test for continuous and categorical data, respectively.
A generalized linear mixed model with logit link was applied to evaluate factors associated with delirium on admission, including variables selected a priori (sex, age, functional disability, dementia, number of chronic diseases, use of CPAP, nutritional status, chest X‐ray or CT findings, and serum CRP). Center was included as random effect.
The crude cumulative incidence of in‐hospital mortality was estimated by Aalen‐Johansen, accounting for the competing event discharge; in‐hospital mortality was then compared between patients with and those without delirium using the Gray test. To evaluate the association between delirium on admission and in‐hospital mortality we applied a multivariable Cox regression model stratified for center and including potential confounders selected a priori (sex, age, functional disability, dementia, number of chronic diseases, use of CPAP, nutritional status, chest X‐ray or CT findings and serum CRP). The proportional hazard assumption was fulfilled and no interaction between delirium and other factors was found.
A sensitivity analysis was conducted excluding the center without m‐RASS evaluation and the largest sample size (i.e., Center 3) to test the robustness of the results against possible misclassification of delirium in this center.
Due to missing values in predictors (functional disability 3.3%, use of CPAP 1.6%, nutritional status 26%, serum CRP 0.2%), we performed multiple imputation using the chained equations (MICE) method, assuming data were missing at random. Ten imputed datasets were created using all the variables in Table 1, as well as outcomes and other partially available information (i.e., other hematological variables, maximum ventilator support during hospital stay, nursing home resident/need for caregiver, respiratory frequency on admission).
Table 1.
Patients' Characteristics on Admission by Delirium Presence
| Characteristics on Admission | Total (n = 516) | No Delirium (n = 443) | Delirium (n = 73) | P‐value |
|---|---|---|---|---|
| Age (median (IQR)) | 78 (73, 84) | 77 (72, 83) | 84 (79, 88) | <.001 |
| Male gender, n (%) | 318 (62) | 284 (64) | 34 (47) | .006 |
| Smoke (current or previous), n (%) | 95 (31) | 84 (31) | 11 (28) | .853 |
| Past or current medical history | ||||
| Hypertension, n (%) | 343 (66) | 293 (66) | 50 (68) | .789 |
| Cardiac diseases, n (%) a | 212 (41) | 176 (40) | 36 (49) | .126 |
| Diabetes mellitus, n (%) | 138 (27) | 118 (27) | 20 (27) | .887 |
| Respiratory diseases, n (%) b | 63 (12) | 53 (12) | 10 (14) | .699 |
| N of chronic diseases (excluding dementia), (median (IQR)) | 2 (1, 4) | 2 (1, 3) | 3 (2, 5) | <.001 |
| Dementia, n (%) | 85 (16) | 50 (11) | 35 (48) | <.001 |
| Malnutrition No, n (%) | 281 (74) | 252 (77) | 30 (54) | <.001 |
| Undernutrition, n (%) | 42 (11) | 23 (7) | 19 (34) | |
| Obesity, n (%) | 58 (15) | 51 (16) | 7 (12) | |
| Drugs (median (IQR)) | 5 (2, 8) | 4 (2, 8) | 6 (4, 8) | .007 |
| Dependence in self‐bathing, n (%) | 84 (35) | 50 (26) | 34 (72) | <.001 |
| Dependence in self‐dressing, n (%) | 77 (32) | 44 (23) | 33 (72) | <.001 |
| Barthel Index (pre‐admission), (median (IQR)) | 100 (79, 100) | 100 (80, 100) | 90 (50, 100) | .019 |
| Functional disability, n (%) c | 171 (34) | 126 (29) | 45 (66) | <.001 |
| Chest X‐ray or CT results, n (%) | .026 | |||
| No opacity | 46 (9) | 39 (9) | 7 (10) | |
| Single opacity | 74 (14) | 56 (13) | 18 (25) | |
| Multiple or bilateral opacities | 396 (77) | 348 (79) | 48 (66) | |
| Use of continuous positive airway pressure, n (%) | 53 (10) | 43 (10) | 10 (14) | .299 |
| Lab serum levels (median ((IQR)) | ||||
| White blood cell count (×109/L) | 7.0 (5.0, 9.7) | 7.0 (5.0, 9.5) | 6.9 (4.9, 10.2) | .88 |
| C‐reactive protein (mg/dl) | 8.7 (4.0, 15.3) | 8.9 (4.3, 15.4) | 7.5 (3.0, 14.5) | .282 |
| Albumin (g/dl) | 3.1 (2.8, 3.3) | 3.1 (2.8, 3.3) | 3.2 (2.8, 3.3) | .975 |
Abbreviations: CT, computed tomography; IQR, interquartile range.
Cardiac diseases included congestive heart failure, coronary heart diseases and atrial fibrillation.
Chronic respiratory diseases include chronic obstructive pulmonary disease and asthma.
Functional disability was defined as the presence of a dependence in bathing or dressing (for Centers 1 and 2) or a Barthel Index ≤90 (Centers 3 and 4).
The results were obtained using SAS (version 9.4) and R (version 3.5.2) software.
3. RESULTS
In total, 553 patients were initially identified, 143 from Center 1, 108 from Center 2, 264 from Center 3, and 38 from Center 4. However, 37 patients were excluded as they had first been admitted to an ICU. The final study sample thus numbered 516 patients. The median age was 78 years (range 65–99), and 318 (62%) were men. Table 1 reports the clinical features of the patients according to the presence/absence of delirium.
Overall, 73 (14.1%, 95% confidence interval (CI) = 11.0–17.3%) patients had delirium on admission: 29 patients (21%) at center 1, 22 (21%) at Center 2, 11 (5%) at Center 3, and 11 (29%) at Center 4. Patients with delirium were on average 7 years older than those without, had an increased number of pre‐existing diseases and were using more prescription drugs on admission. In addition, they had higher prevalence rates of dementia and malnutrition and were more frequently dependent in ADL before hospitalization. However, some between‐center differences were found in the patients' clinical features (Supplementary Table S2).
Table 2 shows the multivariable regression model for the detection of delirium on admission. Dementia (odds ratio (OR) = 4.66, 95% CI = 2.03–10.69), the number of chronic diseases (OR = 1.20, 95% CI = 1.03; 1.40) and chest X‐ray or CT opacities (OR = 3.29, 95%CI = 1.12–9.64 and OR = 3.35, 95%CI = 1.07–10.47, for multiple or bilateral opacities and single opacity vs no opacity, respectively) were significantly associated with delirium. These results were consistent in the sensitivity analysis, i.e., after excluding Center 3 (Supplementary Table S3).
Table 2.
Multivariable Logistic Model on Delirium on Admission After Multiple Imputation (516 Patients 73 with Delirium on Admission). Center Included as Random Effect
| OR (95% CI) | P‐value | |
|---|---|---|
| Sex (male vs female) | 0.79 (0.43; 1.47) | .462 |
| Age (for each year) | 1.01 (0.96; 1.06) | .691 |
| Functional disability (yes vs no) | 1.74 (0.75; 4.03) | .193 |
| Dementia (present vs absent) | 4.66 (2.03; 10.69) | <.001 |
| N. of chronic diseases (excluding dementia) | 1.20 (1.03; 1.40) | .018 |
| Use of continuous positive airway pressure (yes vs no) | 1.44 (0.6; 3.48) | .414 |
| Nutritional status (malnourished vs no) | 1.95 (0.82; 4.64) | .129 |
| Nutritional status (obese vs no) | 1.66 (0.62; 4.42) | .306 |
| Chest X‐ray or CT (multiple or bilateral opacities vs no opacity) | 3.29 (1.12; 9.64) | .030 |
| Chest X‐ray or CT (single opacity vs no opacity) | 3.35 (1.07; 10.47) | .038 |
| C‐reactive protein (for each mg/dl) | 1.02 (0.99; 1.06) | .231 |
Abbreviations: 95% CI, 95% confidence interval; CT, computed tomography; OR, odds ratio.
Overall, follow‐up lasted a median of 19 days; 191 (37%) patients died during hospitalization, while 278 were discharged and 47 were transferred to other hospitals or lower intensity wards.
The crude cumulative incidence of in‐hospital death is shown in Figure 1. At 30 days from admission, there were 145 deaths (mortality 33.8%, 95% CI = 29.3–38.3%) in the no‐delirium group and 38 (53.2%, 95% CI = 41.6–64.8) in the delirium group (Gray test P‐value <.001). At the same time point (30 days), 50.1% (95% CI = 45.3–54.9%) of the patients with no delirium and 24.2% (95%CI = 14.2–34.3) of the patients with delirium had been discharged from hospital.
Figure 1.
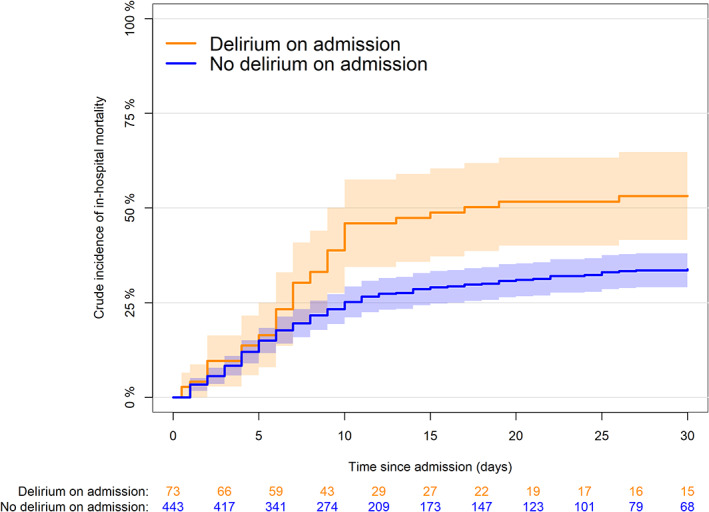
Crude cumulative incidence of in‐hospital mortality by delirium on admission (Gray test P < .0001). Shaded area represents confidence intervals.
In a multivariable Cox regression analysis (Table 3) adjusting for possible confounders, patients with delirium on admission showed an almost two‐fold chance of in‐hospital mortality compared with the ones without (hazard ratio (HR) = 1.88, 95% CI = 1.25–2.83). These results were consistent even after excluding center 3 (HR = 2.00, 95%CI = 1.23–3.25).
Table 3.
Multivariable Cox Model on In‐Hospital Mortality (Stratified for Center) After Multiple Imputation (516 Patients, 191 Deaths)
| Hazard Ratio (95%CI) | P‐value | |
|---|---|---|
| Delirium (yes vs no) | 1.88 (1.25; 2.83) | .003 |
| Sex (male vs female) | 1.39 (1.00; 1.94) | .052 |
| Age (for each year) | 1.05 (1.02; 1.07) | <.001 |
| Functional disability (yes vs no) | 1.32 (0.89; 1.96) | .167 |
| Dementia (present vs absent) | 1.13 (0.71; 1.82) | .602 |
| No. of chronic diseases (excluding dementia) | 1.16 (1.07; 1.26) | .001 |
| Use of continuous positive airway pressure (Yes vs No) | 2.77 (1.83; 4.19) | <.001 |
| Nutritional status (malnourished vs no) | 0.67 (0.39; 1.14) | .139 |
| Nutritional status (obese vs no) | 1.17 (0.76; 1.81) | .481 |
| Chest X‐ray or CT (multiple or bilateral opacities vs no opacity) | 1.85 (0.96; 3.55) | .065 |
| Chest X‐ray or CT (single opacity vs no opacity) | 1.54 (0.75; 3.15) | .237 |
| C‐reactive protein (for each mg/dl) | 1.02 (1.01; 1.04) | .008 |
Abbreviations: 95% CI, 95% confidence interval; CT, computed tomography.
4. DISCUSSION
Our study indicates that delirium occurs commonly in hospitalized older adults with COVID‐19 and carries important prognostic significance. Dementia, pre‐existing comorbidity and pulmonary opacity were significantly associated with the presence of delirium on admission and delirium was significantly associated with increased in‐hospital mortality.
Delirium should be therefore routinely screened for in older adults with COVID‐19 and interventions to improve outcomes should be implemented.
Previous studies evaluating delirium in COVID‐19 patients generally found it to show a high prevalence, ranging from 29% 3 to 42%. 8 , 9 In the only study that focused on patients with pre‐existing dementia, it was 67%. 11 Our results, showing a lower prevalence, partially disagree with the findings of these studies. This difference may be related to the characteristics of the patient samples, to our exclusion of patients initially admitted to the ICU, and to the methods used to assess delirium. Notably, the majority of the above‐mentioned studies did not specify the criteria used to diagnose delirium, 3 , 8 , 9 and two studies failed to state whether delirium was assessed only on admission or throughout the hospital stay. 9 , 11 Our results are, instead, in keeping with those of Mao et al, 25 who found a 14.8% prevalence of impaired consciousness, and Garcez et al, 14 who reported a 12% prevalence of delirium on hospital admission. However, when our analyses excluded the data from Center 3, which diagnosed delirium exclusively on the basis of physician clinical impression, our results concur with those of three recent studies that found delirium prevalence rates of around 25%. 10 , 12 , 13
The observed variability in delirium prevalence between the units participating in our study may be partially explained by differences in physician expertise and methods in delirium detection.
The finding that dementia, pre‐existing comorbidity and pulmonary opacity were significantly associated with delirium on admission was somewhat expected according to previous literature. 5 , 26 , 27 However, a recent study found that age, history of epilepsy, antipsychotic drugs, vasodilators, urea and lactate dehydrogenase, but not dementia and comorbidity were associated with delirium. 15 The present study, thus, adds knowledge in this field.
The prevalence of delirium psychomotor subtypes in our study is also noteworthy. We found that the m‐RASS, where used, gave scores of −1 and below in a sizeable proportion of patients, suggesting hypoactive delirium. This conflicts with what emerged in the only other study that evaluated psychomotor subtypes of COVID‐19‐related delirium, with hyperactive presentations as predominant. 28 Our findings are important in view of the prognostic implications of hypoactive delirium highlighted in non‐COVID‐19 case cohorts. 29 , 30
A further important finding of our study is that delirium was significantly associated with increased in‐hospital mortality. The relationship between delirium and mortality in COVID‐19 has been assessed in very few studies, with inconclusive results. There was no evidence of excess mortality in the study by De Smet et al, 9 while Knopp et al 3 found the opposite. The present study supports the notion that delirium is a marker of clinical severity in older patients with COVID‐19. Our evidence that male gender, pre‐existing comorbidity, use of CPAP, pulmonary opacity and high serum CRP levels were associated with increased mortality is in line with previous studies. 2 , 23 , 24 , 25
Our findings have several clinical implications. First, the finding that delirium prevalence was markedly higher in the centers where delirium diagnoses were based on m‐RASS or DSM‐5 criteria indirectly suggests that all COVID‐19 patients should be screened for this condition using a standardized approach. A second implication concerns the risk factors of delirium. In fact, in light of the results of our study, clinicians should become more suspicious of delirium when patients with COVID 19 are admitted to a hospital unit with either dementia or comorbidity. A third implication regards the prognosis associated with delirium. In fact, the detection of delirium in COVID‐19 patients should prompt the medical team to immediately initiate, whenever possible, treatment of delirium‐precipitating factors and/or to start discussing end‐of‐life decisions with family members. Furthermore, we recommend that specific training in delirium be given to all physicians who may be involved in the care of these patients should the pandemic worsen.
Overall, our findings add to the growing body of literature on delirium in COVID‐19. First of all, it is one of the few multicenter studies 11 , 25 thus far conducted on delirium in these patients. Previous reports concern studies conducted in a single centers 3 , 8 , 9 , 10 , 13 , 14 and/or in smaller samples. 8 , 9 , 13 Moreover, this study also clarifies the factors associated with delirium, which may be helpful for rapid identification of at‐risk subjects. Finally, it strongly supports the role of delirium as an independent predictor of mortality in COVID‐19, an aspect not clearly brought out by previous studies. 3 Another strength of the study is the large sample size and the multicenter design. To our knowledge, it is the largest multicenter study assessing the prevalence of delirium in older COVID‐19 hospital inpatients.
However, the study presents several limitations. First, although delirium was largely diagnosed thanks to the routine use of delirium screening in most of the participating centers, we acknowledge that, given the exceptionality of the period, some cases of delirium may have been missed. However, at the height of a pandemic, it is unreasonable to assume that medical staff will have the time and the state of mind necessary to perform a thorough assessment followed by a non‐pharmacological intervention for delirium management. Second, since the data were collected retrospectively from electronic health records, some missing items (e.g., malnutrition/obesity) may have introduced biases; however, we accounted for this by multiple imputations. Third, the cohorts of patients included in the study were not well balanced between the centers. This, too, may have introduced biases, given that the average delirium prevalence of our population may have been driven mainly by the center with the largest case load. Nevertheless, we performed a sensitivity analysis excluding the center with the largest cohort and also the lowest rate of delirium (Center 3), and the results remained consistent.
In conclusion, delirium was found to be present in about one in seven patients aged 65 years or over, hospitalized with COVID‐19. Dementia, pre‐existing comorbidity and chest X‐ray or CT opacities were significantly associated with the presence of delirium on admission. Furthermore, delirium, along with male gender, age, pre‐existing comorbidity, the use of CPAP as respiratory support and serum CRP on admission were significantly associated with increased in‐hospital mortality.
Supporting information
Supplementary Table S1: Methods to Assess Delirium at Each Study Center During COVID‐19 Emergency.
Supplementary Table S2: Patients' Characteristics on Admission in the Study Centers.
Supplementary Table S3: Sensitivity Analysis on the Multivariable Logistic Model on Delirium on Admission Excluding Center 3.
ACKNOWLEDGMENTS
All researchers who contributed significantly to the work have been listed and a written consent has been obtained from all of them. We are also grateful to the following researchers who helped in collecting data: Drs Gabriella Maria Celora, Valentina Deiana, Nives Ghezzi, Julia Miksza. We also gratefully acknowledge the contributions of the patients, family members, nurses, physicians, staff members, and members of the CoViD‐19 Lombardia Team.
This work has been supported by grants from the Cariplo (Cassa di Risparmio delle Province Lombarde) Foundation, Lombardia Region, Italy.
Conflict of Interest
The authors have no conflicts of interest to disclose.
Author Contributions
Paola Rebora, Alessandro Morandi, Maria Grazia Valsecchi, Giuseppe Citerio and Giuseppe Bellelli designed the study. Paolo Blangiardo, Alice Marchegiani, Andrea Piazzoli, Francesca Mazzeo, Giulia Cesaroni, Anita Chizzoli, Fabio Guerini, Paolo Bonfanti, Alessandro Morandi, Bianca Faraci, Simona Gentile and Giuseppe Bellelli collected the data. Claudio Bna and Giordano Savelli reviewed the X‐ray and CT scans. Paola Rebora and Maria Grazia Valsecchi performed the statistical analyses. Paola Rebora, Alessandro Morandi and Giuseppe Bellelli drafted the manuscript. All authors reviewed the manuscript.
Sponsor's Role
The authors' funding sources did not participate in the planning, collection, analysis or interpretation of data or in the decision to submit for publication. The investigators had full access to the data and were responsible for the study protocol, progress of the study, analysis, reporting of the study and the decision to publish.
REFERENCES
- 1. Leung C. Risk factors for predicting mortality in elderly patients with COVID‐19: a review of clinical data in China. Mech Ageing Dev. 2020;188:111255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Epidemiology for Public Health , Istituto Superiore di Sanità. Characteristics of COVID‐19 Patients Dying in Italy; 2020. https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-analysis-of-deaths.
- 3. Knopp P, Miles A, Webb TE, et al. Presenting features of COVID‐19 in older people: relationships with frailty, inflammation and mortality. Eur Geriatr Med. 2020;30:1‐6. 10.1007/s41999-020-00373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. D'Adamo H, Yoshikawa T, Ouslander JG. Coronavirus disease 2019 in geriatrics and long‐term care: the ABCDs of COVID‐19. J Am Geriatr Soc. 2020;68(5):912‐917. [DOI] [PubMed] [Google Scholar]
- 5. Bellelli G, Morandi A, Di Santo SG, et al. "Delirium day": a nationwide point prevalence study of delirium in older hospitalized patients using an easy standardized diagnostic tool. BMC Med. 2016;14(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morandi A, Di Santo SG, Zambon A, et al. Delirium, dementia and in‐hospital mortality: the results from the Italian Delirium Day 2016, a national multicenter study. J Gerontol A Biol Sci Med Sci. 2019;74(6):910‐916. 10.1093/gerona/gly154. [DOI] [PubMed] [Google Scholar]
- 7. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911‐922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mcloughlin BC, Miles A, Webb T, et al. Functional and cognitive outcomes after COVID‐19 delirium. Eur Geriatr Med. 2020;14:1‐6. 10.1007/s41999-020-00353-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Smet R, Mellaerts B, Vandewinckele H, et al. Frailty and mortality in hospitalized older adults with COVID‐19: retrospective observational study. J Am Med Dir Assoc. 2020;21:928‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zazzara B, Penfold R, Roberts A, et al. Delirium is a presenting symptom of COVID‐19 in frail, older adults: a cohort study of 322 hospitalised and 535 community‐based older adults 2020. Age Ageing. 2020:afaa223. 10.1093/ageing/afaa223. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bianchetti A, Rozzini R, Guerini F, et al. Clinical presentation of COVID19 in dementia patients. J Nutr Health Aging. 2020;24(6):560‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Benussi A, Pilotto A, Premi E, et al. Clinical characteristics and outcomes of inpatients with neurologic disease and COVID‐19 in Brescia, Lombardy, Italy. Neurology. 2020;95:e910‐e920. 10.1212/WNL.0000000000009848. [DOI] [PubMed] [Google Scholar]
- 13. Marengoni A, Zucchelli A, Grande G, Fratiglioni L, Rizzuto D. The impact of delirium on outcomes for older adults hospitalised with COVID‐19. Age Ageing. 2020;49:923‐926. 10.1093/ageing/afaa189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garcez FB, Aliberti MJR, Poco PCE, et al. Delirium and adverse outcomes in hospitalized patients with COVID‐19. J Am Geriatr Soc. 2020;68:2440‐2446. 10.1111/jgs.16803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ticinesi A, Cerundolo N, Parise A, et al. Delirium in COVID‐19: epidemiology and clinical correlations in a large group of patients admitted to an academic hospital. Aging Clin Exp Res. 2020;32(10):2159‐2166. 10.1007/s40520-020-01699-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Emmerton D, Abdelhafiz A. Delirium in older people with COVID‐19: clinical scenario and literature review. SN Compr Clin Med. 2020;2:1‐8. 10.1007/s42399-020-00474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Katz S. Assessing self‐maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721‐727. [DOI] [PubMed] [Google Scholar]
- 18. Lawton MP, Brody EM. Assessment of older people: self‐maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179‐186. [PubMed] [Google Scholar]
- 19. Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol. 1989;42(8):703‐709. [DOI] [PubMed] [Google Scholar]
- 20. Parmelee PA, Thuras PD, Katz IR, Lawton MP. Validation of the cumulative illness rating scale in a geriatric residential population. J Am Geriatr Soc. 1995;43(2):130‐137. [DOI] [PubMed] [Google Scholar]
- 21. Bellelli G, Morandi A, Davis D, et al. 'Validation of the 4AT, a new instrument for rapid delirium screening: a study in 234 hospitalised older people' (vol 43, pg 496, 2014). Correction. Age Ageing. 2015;44(1):175‐175. 10.1093/ageing/afu181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 23. Chester JG, Beth Harrington M, Rudolph JL, Group VDW . Serial administration of a modified Richmond Agitation and Sedation Scale for delirium screening. J Hosp Med. 2012;7(5):450‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rudolph JL. Arousal, attention, and an abundance of opportunity to advance delirium care. J Am Med Dir Assoc. 2016;17(9):775‐776. 10.1016/j.jamda.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 25. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683‐690. 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mazzola P, Ward L, Zazzetta S, et al. Association between preoperative malnutrition and postoperative delirium after hip fracture surgery in older adults. J Am Geriatr Soc. 2017;65(6):1222‐1228. 10.1111/jgs.14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dharmarajan K, Swami S, Gou RY, Jones RN, Inouye SK. Pathway from delirium to death: potential in‐hospital mediators of excess mortality. J Am Geriatr Soc. 2017;65(5):1026‐1033. 10.1111/jgs.14743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morandi A, Di Santo SG, Cherubini A, et al. Clinical features associated with delirium motor subtypes in older inpatients: results of a multicenter study. Am J Geriatr Psychiatry. 2017;25(10):1064‐1071. [DOI] [PubMed] [Google Scholar]
- 29. Bellelli G, Carnevali L, Corsi M, et al. The impact of psychomotor subtypes and duration of delirium on 6‐month mortality in hip‐fractured elderly patients. Int J Geriatr Psychiatry. 2018;33:1229‐1235. 10.1002/gps.4914. [DOI] [PubMed] [Google Scholar]
- 30. Bellelli G, Speciale S, Barisione E, Trabucchi M. Delirium subtypes and 1‐year mortality among elderly patients discharged from a post‐acute rehabilitation facility. J Gerontol A Biol Sci Med Sci. 2007;62(10):1182‐1324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: Methods to Assess Delirium at Each Study Center During COVID‐19 Emergency.
Supplementary Table S2: Patients' Characteristics on Admission in the Study Centers.
Supplementary Table S3: Sensitivity Analysis on the Multivariable Logistic Model on Delirium on Admission Excluding Center 3.


