The angiotensin‐converting enzyme (ACE2) serves as the main entry into cells for SARS‐CoV‐2. 1 ACE2 receptors are found in the central nervous system and angiotensin II is an active product of the renin‐angiotensin system (RAS). 2 Previous studies have implicated the brain RAS in cognitive functions. 3 Recently, neurologic events and delirium have been described in COVID‐19. 4 , 5 However, no previous research has investigated attention performance. A signed informed consent was obtained from the patient authorizing publication.
A 47‐year‐old physician suddenly noticed a persistent difficulty maintaining attention while driving. After 2 h, he developed fever, ageusia, and anosmia. On admission, the patient was awake, alert, and oriented to person, place, date, and situation (AAOX4). He denied psychiatric illness, fatigue, excessive workload, or exposure to any recent traumatic event, such as recent death of a patient, friend, or family member. The Mini‐Mental State score was 30, 6 body temperature 36.6°C, blood pressure 122/68 mmHg, pulse 72 b.p.m., respiratory rate 16 breaths/min, and oxygen saturation 99% (ambient air). Lung auscultation and laboratory tests were unremarkable (Supplementary Appendix). The antigen test for influenza A and B was negative. A high‐resolution computed tomography of the chest was normal (Supplementary Appendix). Nasopharyngeal and throat swab specimens on reverse transcription‐polymerase chain reaction analysis tested positive for SARS‐CoV‐2.
During the disease, the patient remained AAOX4 and without symptoms of depression or anxiety. Although the Mini‐Mental State scores always reached the maximum value, he continued to report ‘difficulties to stay focused’ from Day 1 to Day 10 of the illness. On Days 3, 6, 10, and 16, attentional performance was objectively assessed with the Continuous Visual Attention Test (CVAT; Fig. 1), a go/no‐go task (Supplementary Appendix) that evaluates attention and its subdomains. 7 , 8 Impaired performance is explained by slow reaction times (alertness subdomain); high variability of reaction times, indicating lapses in attention as the test progresses (sustained‐attention subdomain); omission errors (focused‐attention subdomain); and commission errors (response‐inhibition subdomain). The test lasts 15 min, and normative values are available. 6 , 7 , 8
Fig. 1.
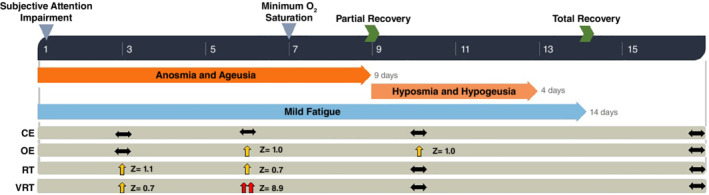
Timeline showing general symptoms and impaired attention functioning. The Continuous Visual Attention Test (CVAT) was used to assess objective attention performance on Days 3, 6, 10, and 16. For each variable of the CVAT, the population mean for the same age and sex of the patient (male, 45 to 50 years old) is set to zero (percentile 50%). The use of a standardized unit (Z‐scores) allows direct comparisons across the different variables. Performance between the 75th and 25th percentiles is considered normal (horizontal arrows). Moderate impairment is defined by performance between the 75th and 95th percentiles (vertical yellow arrows). A value higher than the 95th percentile is considered a severe impairment (double vertical red arrow). On Day 1 of illness, the patient reported a subjective attention impairment. On Day 3, the patient performed worse than the 75th percentile in two subdomains (variability of reaction times [VRT] and reaction times [RT]), indicating a moderate attention impairment. On Day 6, the patient performed worse than the 75th percentile in all variables of the CVAT except commission errors (CE), indicating a severe impairment. VRT is the most affected variable, followed by omission errors (OE). Thus, the sustained‐focused subdomain is the most affected subdomain. Note that the increase in VRT seems to be independent of RT. On Day 10, there was a mild deficit on only one variable (OE). On Day 16, his performance was within the normal range.
On Day 3, the CVAT performance corroborated the patient's subjective attention complaints. He exhibited a moderate attentional impairment in two out of the four attention subdomains as compared to the normative values (males, 45–50 years old).
On Day 6, the patient reported a subjective worsening in his concentration, and the second CVAT was performed. Although his physical examination remained normal, the CVAT performance was worse than the Day‐3 result. He was impaired in three out of the four attention subdomains. As to the sustained‐attention subdomain, he performed above the 95th percentile as compared to age‐ and‐sex matched normative data (a higher percentile indicates a worse performance). Thus, his attentional performance was severely impaired. Eight hours after the worsening of his attentional performance, there was a change in the respiratory status when the patient's oxygen saturation dropped to as low as 94% while breathing ambient air. This illness progression is consistent with previous reports on signs of worsening of respiratory symptoms in the second week after disease onset.
After Day 9, he evolved with clinical improvement. On Day 10, the third CVAT indicated a mild deficit in only one attention subdomain. The response‐inhibition subdomain was always spared, since the patient did not have a clinically significant number of commission errors on any of the test occasions. Moreover, he did not present disorientation, psychomotor and autonomic overactivity, hallucinations, difficulty holding a coherent conversation, somnolence, or decreased arousal. In addition, his mental status examination was always unremarkable. Taken together, we suggest that this patient suffered from a more limited dysfunction involving the attentional system.
On Day 16, he did not report any other symptom, and the CVAT was normal. Then, he was submitted to higher‐level testing using standardized instruments (described in the Supplementary Appendix). Depression and anxiety were measured using the 7‐item Generalized Anxiety Disorder Scale (GAD‐7) 9 and the Patient Health Questionnaire‐9 (PHQ‐9), 10 respectively. The patient's scores did not meet criteria for anxiety (GAD‐7 = 3) or depression (PHQ‐9 = 6). Cognitive performance (Supplementary Appendix) was always above the 75th percentile (memory, visuospatial perception, and executive functions). He was not taking any psychotropic medication.
The key aspect of this case was the decision made by the patient to seek medical help after the attention impairment. A possible SARS‐CoV‐2 infection allowed for prompt isolation. An early attention complaint was the first clinical manifestation. A worsening in attention performance on Day 6 preceded the maximum drop in the patient's oxygen saturation. Attentional deficits may be the first sign and the prodromal stage of respiratory impairments in COVID‐19.
Disclosure statement
The authors have no conflicts of interest to declare.
Supporting information
Appendix S1. Supporting Information.
References
- 1. Millet JK, Whittaker GR. Physiological and molecular triggers for SARS‐CoV membrane fusion and entry into host cells. Virology 2018; 517: 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xia H, Lazartigues E. Angiotensin‐converting enzyme 2 in the brain: Properties and future directions. J. Neurochem. 2008; 107: 1482–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jackson L, Eldahshan W, Fagan S, Ergul A. Within the brain: The renin angiotensin system. Int. J. Mol. Sci. 2018; 19: 876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Helms J, Kremer S, Merdji H et al. Neurologic features in severe SARS‐CoV‐2 infection. N. Engl. J. Med. 2020; 382: 2268–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beach SR, Praschan NC, Hogan C et al. Delirium in COVID‐19: A case series and exploration of potential mechanisms for central nervous system involvement. Gen. Hosp. Psychiatry 2020; 65: 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975; 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 7. Schmidt G, Alvarenga R, Manhães A, Schmidt S. Attentional performance may help to identify duloxetine responders in chronic pain fibromyalgia patients. Eur. J. Pain 2017; 21: 977–986. [DOI] [PubMed] [Google Scholar]
- 8. Simões EN, Padilla CS, Bezerra MS, Schmidt SL. Analysis of attention subdomains in obstructive sleep apnea patients. Front. Psych. 2018; 9: 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: The GAD‐7. Arch. Intern. Med. 2006; 166: 1092–1097. [DOI] [PubMed] [Google Scholar]
- 10. Kroenke K, Spitzer RL, Williams JB. The PHQ‐9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001; 16: 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.


