Abstract
To date, the strongest predictor for dying with COVID‐19 is suffering from several chronic disorders prior to the viral infection. Pre‐existing multimorbidity is highly correlated with socioeconomic inequality. In turn, having several chronic conditions is closely linked to multiple medication intake, especially in richer countries with good access to biomedical care. Owing to its vertical structure, biomedicine often risks giving multiple treatments in an uncoordinated way. Such lack of integrated care can create complex forms of iatrogenic harm. Multimorbidity is often exacerbated by a pharmaceuticalization of social deprivation in place of integrated care. In this article, I explore the possibility that clusters of over‐medication are a contributing factor to higher death rates from COVID‐19, especially in poorer areas within richer countries. Anthropological perspectives on the social embeddedness of multimorbidity and multiple medication use can expand our understanding of who is most vulnerable to SARS‐CoV‐2.
Keywords: COVID‐19, multimorbidity, social inequality, polypharmacy, iatrogenesis
”We Really Need to Get Data and We Need to Get Data Fast”
On March 18, 2020, Dr. Anthony Fauci and Dr. Howard Bauchner discussed a possible link between hypertension medications and a heightened risk of dying with a coronavirus infection. Bauchner is the editor of JAMA, the journal of the American Medical Association. Fauci directs the U.S. National Institute of Allergy and Infectious Diseases. After joining the White House Coronavirus Task Force, Fauci became the world's most famous scientific advisor on COVID‐19. In this conversation, Fauci highlighted possible links between ACE (angiotensin converting enzyme) inhibitors and COVID‐19 fatalities. Fauci said that ACE inhibitors can increase “the expression of the receptors for the virus” (JN Learning 2020). Fauci was struck by reports from Italy that the vast majority of those who died with COVID‐19 suffered from hypertension. Italy—then the European epicenter of the pandemic—was a rich country with excellent access to medical care, and many COVID‐19 victims had been taking ACE inhibitors to treat their hypertension. “Why should someone who has hypertension that's well controlled have a much greater chance of dying than somebody else with any other kind of underlying condition?” Fauci asked. “We really need to get data and we need to get data fast” (JN Learning 2020). Fauci's concern is about both the clinic and the population. Significant patterns on a population level (here, Italian death rates) flag up patterns in clinical practice (widespread use of ACE inhibitors), which then lead to an exploration of pathogenesis (ACE inhibitors, cell receptors). I agree with Fauci that a careful triangulation of pharmacological, clinical, epidemiological, and social science evidence is necessary to understand the action of the virus, how it spreads, and who is most vulnerable to it.
In the months following Fauci's call for data, a handful of medical articles appeared that examined the link between COVID‐19 and antihypertensive drugs. The results were said to be inconclusive because none of the studies “adjusted for confounding variables” and so it remained unclear “if this association [between antihypertensives and high death rates] is related to the pathogenesis of hypertension or another associated comorbidity or treatment” (Patel and Verma 2020). The American Heart Association, a nonprofit organization that funds cardiological research and patient education, continued to recommend ACE inhibitors. In the absence of certainty about increased risk from ACE inhibitors, they told patients: “Continue taking all your medications as prescribed, including ACE inhibitors. … If you are diagnosed with COVID‐19, you should be fully evaluated before adding or removing any treatments … your overall medical condition is much better if your blood pressure and diabetes are optimally controlled” (AHA 2020).
Fauci's call that “we need to get data fast” was only heard for ACE inhibitors but not for other medications, and possible links between the pathogenesis of COVID‐19 and complex medication patterns remain entirely unknown. I argue that Fauci's challenge remains as important now as it was at the beginning of the pandemic. There are at least two ways that chronic pharmaceutical use might put people at a higher risk of dying with COVID‐19. One is that medications could directly augment virulence and pathogenesis (as intimated by Fauci). Another is that overtreatment adds to the overall morbidity index, regardless of any specific drug mechanism, thus making an overmedicated patient less healthy and less able to resist succumbing to the disease. In what follows, I will explore the possibility that multiple medication intake may lead to poor outcomes for coronavirus by this pathway. My goal is to show how medical anthropology, especially an anthropology of pharmaceuticals, may add a less obvious path in explaining the global impact of COVID‐19 than that taken by virologists or epidemiologists.
While the pandemic modeling first anticipated that the coronavirus would behave like influenza, researchers realized that COVID‐19 is a “complex multi‐system clinical syndrome” (Roberts et al. 2020) that has a completely different trajectory than influenza. According to clinical reports as of this writing, the virus starts to spread in the lungs but can go on to attack all other organs, including the brain. Even when a patient survives the virus infection, the long‐term effects, such as cognitive impairment, psychological distress, or reduced lung capacity, can be severe and protracted. Some of the long‐term harm may even be caused by “iatrogenic damage” (Roberts et al. 2020), which comes from clinical uncertainty over how to best treat those with severe COVID‐19 symptoms. It is now better understood that treating COVID‐19 as if it were a form of pneumonia (e.g., by connecting all patients with low oxygen levels to ventilators), may do more harm than good (Marini and Gattinoni 2020). The pathogenesis I will explore here is the one that connects victims’ multiple chronic health problems to the risks of dying with COVID‐19, specifically because of victims’ multiple chronic medication histories. Could it be that socioeconomic deprivation coupled with chronic medication consumption is a predictor of dying with a SARS‐CoV‐2 infection?
To date, one medical study has appeared that shows polypharmacy being strongly correlated with the risk of catching Sars‐CoV‐2. In an analysis of half a million U.K. biobank users, McQueenie and colleagues found “that there was a clear dose relationship whereby the risk of a COVID‐19 positive test rose steadily with polypharmacy level” (2020: 15). This study did not identify which kinds of medications were used, and it only explored infection risks.
My approach picks up on these threads from the medical literature to explore social factors linked to the spread and impact of the pandemic. First, I explore the social causes of multimorbidity and overmedication in relation to socioeconomic deprivation in one of the U.K.’s most deprived areas where I recently conducted fieldwork. I then offer case studies that demonstrate how multiple medication use exacerbates patients’ health. In the final section, I explore population‐level links between chronic illness, chronic medication use, and dying from an acute coronavirus infection. My suggestion is that medication patterns should be considered in studies on pathogenesis and epidemiology. COVID‐19 death rates seem to be highest (1) where a country has high rates of multimorbidity; (2) where multimorbidity is deepened by high levels of social inequality; (3) where the health system enables easy access to multiple medications; and (4) where multiple chronic treatments are insufficiently integrated. In wealthy countries, COVID‐19 appears to claim most victims in poorer pockets within richer regions, and complex medication regimes might be a missing piece in this puzzle. Anthropological perspectives might offer novel ways of conceptualizing not just the social but also the epidemiological contours of the pandemic as problems of “local biologies” (Lock and Nguyen 2018).
Uncovering Multimorbidity
The clinical and epidemiological findings on COVID‐19 and multimorbidity are, by now, fairly robust. From early on, medical reports found strong links between underlying health conditions and COVID‐19. The majority of people infected with SARS‐CoV‐2, by some estimates 78%, remain asymptomatic (Day 2020). A minority of infected people experience mild to moderate flu symptoms. In perhaps 1% of people infected, COVID‐19 turns fatal. Whether a SARS‐CoV‐2 infection kills seems highly correlated to the presence of pre‐existing problems. The origins and forms of multimorbidity are thus extremely important for understanding global COVID‐19 mortality patterns.
Already the first studies of mortality in China's Wuhan city showed that almost all the people who died with the virus had pre‐existing disorders (The Novel Coronavirus Response Team 2020). The data from China highlighted heart disease, diabetes, chronic respiratory disease, high blood pressure, and cancer (The Novel Coronavirus Response Team 2020). Studies from around the world confirmed these findings. A study among patients admitted to New York City hospitals (Richardson et al. 2020) found similar conditions, especially for patients with hypertension (56.6% of COVID‐19 patients), obesity (41.7%), and diabetes (33.8%). Twenty‐one percent of the COVID‐19 patients admitted to NYC hospitals died. Using an assessment tool called the Charlson Comorbidity Index (CCI), which predicts 10‐year survival rates in patients with multiple chronic problems, this study found that among all the patients admitted to NYC hospitals with a SARS‐CoV‐2 infection, half were expected to die of existing morbidities within the next 10 years.
Forensic pathologists in Hamburg, Germany, performed full autopsies on hundreds of COVID‐19 victims. In every case, they found one or more of these pre‐existing conditions: heart disease, asthma, chronic obstructive pulmonary disease, peripheral artery disease, diabetes, obesity, or a neurodegenerative disease (Wichmann et al. 2020). The Hamburg study discovered at least one pre‐existing condition in every patient examined, and multimorbidity in the majority of cases. The findings from Hamburg were widely reported in German media. Dr. Klaus Püschel, director of the Hamburg Institute for Forensic Medicine, argued that SARS‐CoV‐2 is not nearly as serious a threat to the general population as it was believed to be. No one dies only of a SARS‐CoV‐2 infection, he said. At least one underlying health problem is always present (Wunder 2020).
Multimorbidity occurs when the same person suffers from two or more chronic disorders, which can be noncommunicable, infectious, or mental (Academy of Medical Sciences 2018). There is no agreed way of how to measure multimorbidity: Some researchers classify multimorbidity by how many disorders occur together, while others look for systematic clusters among co‐occurring problems. What problems are counted in one of these clusters also varies, some researchers consider only a handful of chronic disorders as constitutive of multimorbidity (Dugravot et al. 2020), while others capture dozens of conditions (Payne et al. 2020). Multimorbidity overlaps with other constructs, such as frailty (Hanlon et al. 2018) and disability (Stineman et al. 2012).
The notion of “comorbidity,” as the coexistence of an “index” disorder and a secondary disorder, emerged in the 1970s (Weaver et al. 2016). Research into multimorbidity as a more complex category of diagnosis was almost nonexistent a decade ago but took off when the epidemic spread of co‐occurring chronic disorders came into view (Busija et al. 2019: pp. 1025). Rising rates of multimorbidity have become a concern for both clinicians and epidemiologists. In rich countries, multimorbidity makes up 25–50% of the overall disease burden (Garin et al. 2016; van der Aa et al. 2017). In the United Kingdom, for example, multimorbid conditions account for 50% of GP appointments, 64% of outpatient appointments, 70% of inpatient bed days, and 70% of total health expenditures (Aiden 2018: 4). Longer lifespans mean more multimorbidity: The older people are, the more chronic health problems they have. Up to two‐thirds of people over 65 are multimorbid. Treating older patients accounts for the majority of all health expenditures (Kaufman 2015).
Multimorbidity is strongly correlated with protracted medication uses. About half of older adults in richer countries are taking five or more medications (Mangin and Garfinkel 2019: 1). The pharmaceutical industry promotes the regular consumption of five or more medications as necessary for the maintenance of health. For the industry, chronic polypharmacy is recommended as the new normal because multimorbidity is now normal. Already a decade ago, people in richer countries were on nine–13 prescription drugs in any given year (Dumit 2012: 2). Regimes of chronic polypharmacy originated in the United States in the 2000s but have become normalized across the world wherever drugs are affordable and accessible.
Multimorbidity is not a new condition, as there have always been people with more than two health issues at the same time. But the growth of multimorbidity diagnoses and the biomedical focus on it are new. Dr. Chris Whitty, the U.K. government's chief advisor on COVID‐19, argued in January 2020—just when the pandemic hit—that multimorbidity did not come into view for so long because biomedicine is organized “vertically” along specific diseases, while a “horizontal” understanding of simultaneous disorders is lacking (Whitty et al. 2020). Verticality increases the risk of iatrogenesis by way of multiple uncoordinated treatments. As others have shown, this verticality is itself an artefact of reliance on a biomedical model that tries to isolate causes of disorders and to develop therapies that are reductionistic (White 2006: 141–42). Although multimorbidity is a recognized and proliferating problem, research on the interactions between multimorbidity and protracted medication use is still largely absent.
Current biomedical research on multimorbidity could be of use in mapping possible research avenues in relation to COVID‐19. Some of this explores nonrandom clusters of disorders (Academy of Medical Sciences 2018: 5; Busjia et al. 2019). Other research explores how socioeconomic inequality congeals and expands chronic disease clusters. The most‐cited study on multimorbidity is by Barnett and colleagues (2012). In this study, Scottish patient health records were scrutinized and showed a strong correlation between socioeconomic deprivation and multimorbid suffering. A decade ago, already 23.2% of all Scottish patients were multimorbid, and a third of these multimorbid patients had both mental and physical problems. Socioeconomic deprivation shifted the onset of multimorbidity forward: “Young and middle‐aged adults living in the most deprived areas had rates of multimorbidity equivalent to those aged 10–15 older in the most affluent areas” (Barnett 2012: 39). In deprived neighborhoods, people in their 40s and 50s are as likely to have multiple chronic problems as 60 or 70 year olds in the average population. Barnett's (2012) quantitative results tally with qualitative studies on GPs’ “endless struggle” with multimorbidity in deprived areas (Lawson et al. 2013; O'Brien et al. 2011). All available data suggest that multimorbidity keeps rising.
Even when multimorbidity is recognized by clinicians, there are few therapeutic protocols to deal with it (Guthrie et al. 2012). Given the logic of pharmaceutical industries, multimorbidity will continually escalate the number of pills required for treating the average patient. The more a health system is compartmentalized by specialization, the more likely it is that multimorbidity goes unrecognized as a problem of its own. Patients with multiple chronic problems experience biomedical care as lacking coherence (Schiøtz et al. 2016).
Some of the most important work in anthropology on multimorbidity is on syndemics, which are “aggregation[s] of two or more diseases or other health conditions in a population in which there is some level of deleterious biological or behaviour interface that exacerbates the negative health effects of any or all of the diseases involved” (Singer et al. 2017: 941). The syndemics approach “move[s] beyond common medical conceptualisations of comorbidity and multimorbidity” (Singer et al. 2017: 941) by emphasizing structural violence (Farmer 1996) and social suffering (Kleinman et al. 1997). Syndemics researchers identify a number of multimorbid cluster patterns. For example, Mendenhall (2012, 2016) describes a pattern of “violence, immigration, depression, type 2 diabetes, and abuse” among Mexican immigrant women living in the United States. Immigration tears families apart and leads to social isolation. Depression is conditioned by traumatic childhood experiences and diminished self‐efficacy. Diabetes is co‐constituted by gender roles and food preferences. Structural violence includes living in unsafe neighborhoods. Limited access to health care is regarded as another form of structural violence.
A syndemics perspective helps connect COVID‐19 as “complex multi‐system clinical syndrome” to multimorbidity, polyiatrogenesis, and social deprivation. In the next section, I will describe how people in a poor area of the United Kingdom experience multimorbidity and polyiatrogenesis, pointing to an as yet little explored syndemic pattern and also to possible ways we might rethink social vulnerability to SARS‐CoV‐2.
Overtreating the Underprivileged
My research took place in one of the U.K.’s most deprived areas, which I will call Greyfield. Similar to the pattern described by Mendenhall (2012, 2016), Greyfielders experience clusters of structural violence, depression, diabetes, and abuse. However, they were not immigrants and they had universal access to health care, provided free of charge by the National Health Service (NHS). Thus, even the poorest are frequently on multiple medications without any financial burdens on households.
The population of Greyfield is largely White British. Most of the housing is made up of apartments in tower blocks and low rises built in the 1960s and 1970s. The area saw substantial redevelopments in the 1990s and 2000s. Despite some improvements, Greyfield still has some of the U.K.’s worst scores for health, income, employment, education, and crime. Life expectancy of area residents is shockingly low at only 61 years on average, which is 20 years shorter than the average U.K. life expectancy. Greyfield's life expectancy is below that of Zimbabwe.
One of the infrastructural improvements to Greyfield since the 2000s was the construction of Greyfield Care Centre (GCC), a joint facility of the NHS and the local council. GCC tries to take a more integrated approach by combining different medical specializations (primary care, cancer support, dentistry, midwifery) with social services. GCC also houses a clinic for Lifestyle, Nutrition, and Complementary Therapy (LNC). My fieldwork was conducted in the LNC section of GCC. I analyzed 80 case files, discussed 30 selected cases with therapists, and shadowed 40 consultations. Most of the patients get referred to LNC by GPs in GCC. The patients that the LNC practitioners see are the same as those treated by the GPs, but LNC consultations allow far more time for patients to talk about their lives, their diets, and their medication regimes. LNC's hour‐long, open‐ended consultations allowed patients to explore complex problems in much greater depth than in the GP consultations, where patients were given no more than five or 10 minutes. The average length of GP consultations in the NHS—nine minutes—is one of the lowest among OECD countries (Wardle 2019). In the NHS, patients’ time with GPs is extremely rationed, and longer consultations with a doctor are unavailable.
In LNC, Greyfield patients presented with a wide range of problems, but a few constellations, came up repeatedly. Five or more co‐occurring problems was the norm. The majority had mental problems along with physical problems. Traumatic life events were common. Chronically difficult social circumstances were nearly always present. Among various physical complaints, digestive diseases were virtually always present. Obesity was common and was usually coupled with diabetes. Hypertension was pervasive. Physical problems appeared together with mental problems. Depression and anxiety were the most common mental illnesses.
Both mental and physical problems were often related to life trauma. Many traumatic events happened in relation to family members. Domestic violence and emotional abuse in intimate partnerships were common. Loss of family members, often at a young age, was another frequent source of trauma in this population. Miscarriages were also common. Haunting memories of losses suffered were a recurrent problem. Susan, a woman in her early 50s, for instance, first came to LNC because she wanted to lose weight and sleep better. During the first consultation, she broke down in tears. The sleep disturbance had started the previous year, when her brother died and one of her surgeries went wrong. She said her family was broken: Mother, father, cousins, uncles had all died, and her twin sister died when she was still a child. She said she often wakes up at night in a panic, as if a bright light was shining into her eyes.
Substance use was mentioned often by patients. Mostly, this referred to alcohol (for patients of all ages) and cannabis (among younger patients). Feeling “empty” or bored were patients’ explanations for why substances were used. Strong food aversions were also common. For example, Jack, a patient in his late 30s, complained about depression, panic attacks, acid reflux, sleep disturbance, and headaches. He also suffered from severe back pain, which two previous surgeries failed to make better. He traced his problems to his childhood: He was always disgusted by eating meat, but his parents forced it down his throat. In his experience, he said, the best remedy against the pain and the panic is smoking cannabis. Patients like Susan and Jack presented with complex multimorbidity in ways that are probably similar to many clinics in the world where a combination of poor diet, precarity, and multiple social traumas coalesce to cause or augment physical suffering.
Despite the common presentation of patients at Greyfield, and despite the ample access to care they were provided, their overall state of health seemed often worsened by uncoordinated treatments. Molly's experience exemplifies this vividly. Molly was a 37‐year‐old woman born and raised in Greyfield. Molly came to LNC for a persistent pain in her foot. In the first consultation with Heather, the LNC practitioner, Molly added that she was also suffering from type II diabetes, that she wanted to lose weight, and that she needed help with her irritable bowel syndrome (IBS). Molly was aware of the main food triggers for her IBS, but she could not keep it in check. Molly said that her job as a care worker was stressful, especially when working late hours. She said her stress “gets to my stomach.” Her bowel movements were very irregular. The first time she was diagnosed with IBS was when she was a child. She got some treatment, and the problem was not too troubling until five years ago when she had a protracted and difficult breakup with her husband, during which she and her son suffered domestic abuse. That led, in turn, to lots of behavioral problems in her son that she was still struggling with. Asked by Heather if she had time left to look after herself, Molly said “my self‐care is really bad, I know that it is really bad.”
Molly took a dose of Imodium every morning and had become completely dependent on it. Imodium (loperamide) is a common over‐the‐counter drug for acute diarrhea. The medication slows down the digestive process and should not be used for more than 48 hours. Molly complained about her daily eating habits, especially too many potato chips: “Pringles are my downfall.” Molly drank three–four liters of Coca‐Cola throughout the day: “I am really bad with fizzy drinks.” Asked if the GPs had explained the links between diabetes, blood sugar and high‐sugar drinks, Molly said that the doctors did not yet have time to talk about food. Instead, they were “just firing tablets at me.” She had not been told by anyone face to face how to manage her diabetes. The LNC practitioner asked about Molly's blood sugar readings, but Molly replied that she had no idea. Heather fetched a blood sugar kit and administered a test, by pricking one of Molly's fingers and putting the blood in a glucose monitor. The reading showed an extremely high glucose level of 17 mmol/L. Heather asked what medications Molly is on. Molly pulled a black rubbish bag out of her handbag and put it on the table: “My lucky bag of medicines,” she laughed. Molly said that she is taking two medformin (a standard tablet for diabetes) every morning, plus another diabetes medication. Heather said that Molly should find out how to monitor her blood sugar levels, not least because her levels are high despite already being on drugs.
Molly unpacked seven other drugs from her “lucky” bag: an antidepressant (citalopram 20mg); propanonol (a beta‐blocker, against the anxiety of having diarrhea while out of the house); Fianola, a contraceptive; an antihistamine for allergies; omeprazole against gastric reflux; gentamicin, an antibiotic drug; and a high‐dose ibuprofen for the pain in the foot. Molly continued to unpack a number of alternative remedies, including sage pills and matcha green tea pills, which were meant to help her against hot flashes. She said that she is a “hot person,” “burning up very quickly,” and joked that she should “move to Iceland” to feel more comfortable.
Heather asked about Molly's body weight. She said she weighs about 20 stone. At her body height, her BMI was 45, putting her into the obese category. Molly said she has always been “big.” She then asked about a growth on her foot and showed it to Heather. A GP had told her it was a ganglion. It caused her much pain and restricted her movement. By overcompensating with her other foot, she developed plantar fasciitis and dislocated a bone. She had phoned her GP about the ganglion but was told that there was nothing else they could do. Heather probes further about what kind of pain Molly has in her foot and is told it is like “pins and needles,” which Heather said might be related to the diabetes. Molly further said that she has not been told by any doctor what she could do about the plantar fasciitis. Drawing the consultation to a close, Heather said she does not want to prescribe any therapy yet. Instead, Molly should come back for a full assessment and then they would work out a care plan. Molly thanked Heather for taking so much time, saying that GPs only ever spoke to her briefly and only about a specific ailment. Heather went on to add that Molly should stop drinking fizzy drinks immediately. Molly asked if juices or fizzy water would be alright to have instead, but Heather told her that neither of them would be good for her, because of both the sugar and the fizz.
Molly's case gives an insight into the “invisible epidemic” (Mangin and Garfinkel 2019) of patients with multiple health problems receiving multiple poorly integrated treatments that too often cause more harm than good. My point is not that Molly's case is exceptional, but rather that it is not exceptional at all. In fact, the cluster of multiple comorbidities experienced by Molly could be said to appear in syndemic form in many poor pockets of rich nations around the world. These pockets reveal high levels of multimorbidity and polyiatrogenesis. What I am suggesting is that such areas of social deprivation and overmedication might be at a substantially higher risk from a virus that becomes particularly dangerous for people with multiple chronic conditions.
Molly's overflowing bag of medicines is an example of how multimorbidity is deepened by the use of too many pharmaceuticals. I am not arguing that any single one of the GPs’ treatments is irrational. What I am arguing is that a vertical system does not give physicians enough time to control for potentially iatrogenic effects, especially in chronic patients. When multiple single‐target medications are coupled with a lack of explaining what is going on, the risks of overtreated ills become even greater. In an era of rising multimorbidity, biomedical specificity becomes part of a new kind of pathology.
What is so striking about multimorbid patients in the United Kingdom is that their poor health cannot be attributed to a biomedical treatment gap, in the way that this might be done in Mendenhall's (2012) study on syndemics and structural violence in the United States. The U.K. situation also differs from places like Brazil, where poverty is heavily pharmaceuticalized (e.g., Béhague 2015; Biehl 2005; Scheper‐Hughes 1992). In the United Kingdom, GPs are too short of time to treat complex patients in complex ways. They throw a pill at any ill in the belief that this constitutes good care while being unable to deal with the underlying problems and while losing sight of iatrogenesis. Greyfielders are deprived socioeconomically, but this deprivation does not translate into a lack of health care. The problem is a lack of integrated care.
Pharmaceuticalized Pockets within Richer Regions
It is still early to know the links between multimorbidity, polyiatrogenesis, and COVID‐19. As mentioned, there is not a single medical study yet on links between COVID‐19 fatalities and multiple chronic medication uses. No one is tracking systematically what kind of medications COVID‐19 victims are consuming when they get into hospitals with severe symptoms, let alone how chronic medication regimes interact with COVID‐19 in the community setting.
Even without this clinical evidence, it is possible to look for patterns on a population level. In the U.K. clinic I studied, high levels of multimorbidity and polyiatrogenesis were evident. If the hypothesis of chronic polypharmacy as a risk factor for COVID‐19 holds, we would see higher rates of COVID‐19 deaths in countries with high rates of multimorbidity, social inequality, ready access to biomedicine, and pronounced verticality in service delivery. What I found through ethnographic work in a U.K. clinic would lead to expect that death rates are indeed higher in the United Kingdom than in countries where this syndemic pattern is not as pronounced.
The global epidemiology of COVID‐19 is still in flux, and many puzzles still have no convincing solution. Nevertheless, some epidemiological patterns have crystallized and remain fairly stable. First, we now know that the United Kingdom had become one of the world's hardest‐hit countries by May 2020. While it is still difficult to draw meaningful comparisons between countries, there is solid evidence that the United Kingdom was exceptionally affected by the pandemic. By August 2020, the United Kingdom had one of the world's highest COVID‐19 death rates, both by absolute numbers of victims (more than 50,000) and by fatalities per million population (685 compared to 94 in the world) ( Our World in Data 2020). The United Kingdom started to record “extremely high” excess death rates from week 13 onward (EuroMOMO 2020). While most other European countries saw their excess death rates return to normal levels over the next three weeks, and while other nations saw their case fatality rate decline as more people tested positive without deadly outcomes, the United Kingdom continued to record extremely high excess rates for another eight weeks. It took two months for the U.K.’s nationwide lockdown to bring a significant reduction in death rates. In June, a full 12 weeks into lockdown, the United Kingdom recorded daily death rates higher than all other EU countries combined. The United Kingdom has done worse than most countries in the world, including the United States. There were abject errors in the U.K. government's COVID‐19 response (Horton 2020), but these policy failures cannot explain the U.K.’s exceptionally high death toll.
Studies on European death rates consistently found correlations between social deprivation and dying with the virus: “COVID‐19 is painfully exposing the existing and persisting health inequalities in our societies. This pandemic will have the heaviest impact on the lives of people living in deprivation or facing difficult socio‐economic circumstances” (EuroHealthNet 2020). The United Kingdom showed the same pattern. Across England, Wales, and Scotland, death rates were more than twice as high in socioeconomically deprived areas than in other areas. People in low‐paid employment were dying at much higher rates than those in white‐collar employment (New Statesman 2020). Socioeconomic inequality is one of the strongest predictors for dying with COVID‐19 (Clark and Whiteley 2020).
In the United Kingdom, as in practically all the European countries, social inequality does not translate into unequal access to biomedical care. In Greyfield and elsewhere in the United Kingdom, relative poverty does not mean less access. But no amount of clinical care can shield patients from dying with COVID‐19. Docherty and colleagues’ (2020) study on hospital outcomes in the United Kingdom found that the death rate of all patients admitted was 33%. In ICU units, 45% died. The United Kingdom is not an outlier in poor outcomes of hospital care. Reflecting on her gruelling experiences of giving intensive care to COVID‐19 patients in NYC hospitals, Dr. Helen Ouyang (2020) realized that all the biomedical care in the world would not save people's lives:
What I think will actually cause moral injury is seeing people die after getting the most advanced care available. People who come in talking, with stories to share. They get care—the best that modern medicine has to offer—with life‐prolonging machines and IV drips of all sorts of critical‐care drugs. We put our full minds and whole hearts into trying to save them. Then I see their bodies shut down anyway.
Even the best‐equipped health care system offers no good defense against COVID‐19.
Compared to other infectious diseases, COVID‐19 behaves in an utterly surprising way. All other major infectious diseases—tuberculosis, HIV, or malaria—show a strong correlation between low GDP and high death rates. SARS‐CoV‐2 inverses this pattern: So far, the impact of the virus was far more severe in rich countries than in low‐income countries. To be sure, the pandemic is still unfolding and what the situation might be in the long run is not known. Yet, to date, high GDP and high COVID‐19 death rates are strongly correlated. By mid‐August 2020, upper‐middle and high‐income countries suffered 90% of all COVID‐19 deaths, despite having about half the world's population. This pattern is so strange because infectious diseases usually strike much harder in poorer countries (see Figure 1).
Figure 1.
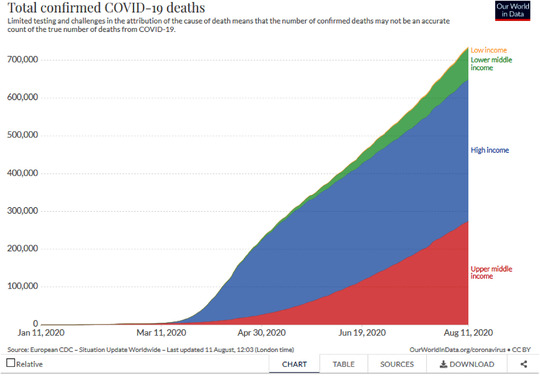
Total confirmed COVID‐19 deaths by country GDP. [This figure appears in color in the online issue]
Until July 2020, all the world's countries with the highest per million population death rates lay in a corridor stretching from Northwest to Southwest Europe. Sweden, a country famous for not implementing a lockdown, only joined this group in the last days of May. Death rates per million population of 500 or more outside these European countries have so far only been reached in the United States, Chile, and Peru, and that only since July. Viral infections usually take advantage of “multiple co‐infections and biological and social vulnerabilities in a world where syndemics disproportionately affect marginalized and less‐resourced communities” (Manderson and Wahlberg 2020: 2). That means, almost without exception, that infectious diseases are far worse in poorer countries than in richer countries. COVID‐19 turns this pattern on its head: All the hardest‐hit countries are GDP‐rich countries. COVID‐19 does not seem to strike most where levels of absolute poverty are high, instead it hits the rich countries. There were some outliers, notably East Asian countries did far better than what their GDP per capita would lead to expect. The overall trend is clear, however. The world map of COVID‐19 mortality does not show any Global North/South distributions along poor access to quality health care. Instead, the COVID‐19 map looks like an atlas of rich industrialized countries with excellent clinical care. To be sure, GDP is a poor proxy for ease of access to biomedical treatments—U.S. health care is a case in point. At the very least, it is safe to say that high income and excellent biomedical care do not protect against high COVID‐19 death rates.
The country‐to‐country comparison hides that relative deprivation within a wealthy context is a key risk factor for health (Ecks In press). COVID‐19 strikes poorer pockets within richer regions. Relatively deprived communities do not necessarily lack access to biomedical care. These communities may have high levels of multimorbidity within a context of relatively good health care access. Good access to health care clearly does not protect anyone from dying with COVID‐19. In fact, easy access may translate into chronic overmedicalization, which, in turn, may make people more vulnerable to dying with the virus infection. The U.K. data on double mortality rates in poorer areas confirm this pattern. Other countries with stark regional and local wealth disparities exhibit this pattern as well. Relative deprivation leads to higher rates of multimorbidity. Multimorbidity increases the risk of polyiatrogenesis, especially where treatments are accessible and affordable. It is possible that COVID‐19 particularly affects multimorbid patients who are prematurely sick because of deep wealth inequalities and whose multimorbidity is exacerbated by polyiatrogenesis.
It is possible that there are other factors at work that explain why GDP‐rich countries have much higher death rates. Some of them are about GDP and global connectedness; others are about GDP and population age structure. SARS‐CoV‐2 probably first made impact along global air traffic routes, so that many poorer countries might have had a delayed exposure to the virus. But date of exposure seems unrelated to eventual death rates. Thailand, for example, was the first country outside China to record a SARS‐CoV‐2 infection, on January 13, 2020. The country has a population of 69 million. By August, it had 58 deaths. The U.K. population is 66 million. The country recorded its first SARS‐CoV‐2 case on February 28, 2020, seven weeks after Thailand. By August, the United Kingdom had more than 50,000 deaths. Per one fatality in Thailand there were 862 fatalities in the United Kingdom. Well into June 2020, the United Kingdom recorded five times more daily deaths than Thailand's entire death count in a population larger than the United Kingdom's.
Many argue that richer countries, especially in North–Southwest Europe, became the global epicenter of the pandemic because people there have longer life expectancies and, therefore, higher rates of multimorbidity. But longer life expectancies alone cannot explain any of these patterns. Among the Europeans, only Italians and Spaniards are in the top 10 globally. The United Kingdom ranks only 29th in the world for life expectancy. People in Japan, Hong Kong, and many other countries have much longer life expectancies than those in the United Kingdom, but are not nearly as much hit by the pandemic. The same point can be made about a population's median age as a protective factor against high COVID‐19 death rates. That low‐income countries have younger populations and “therefore” have lower COVID‐19 death rates is taken as an obvious fact (e.g., Pulla 2020). But if median age were a vital factor, why would Peru have a COVID‐19 death rate of 651 per million population at a median age of just 27.5 years? At 46.3 years, Japan has the world's highest median age; at eight deaths per million population, Japan also has one of the world's lowest COVID‐19 death rates. Neither a country's life expectancy nor its median age seem to be decisive factors in COVID‐19 death rates.
To date, none of the available epidemiological and population data can confirm or disconfirm links between high rates of chronic medication uses and COVID‐19 death rates. High GDP alone does not translate directly into high rates of pharmaceutical consumption, fragmented care, or deeper wealth disparities within countries. But the fact remains that the risk of dying with COVID‐19 is not correlated with low income levels, as practically all other infectious diseases are. Nor is it related to poor health care access, as is the case with all other infectious diseases. Instead, epidemiology of COVID‐19 seems to confirm that countries with high rates of multimorbidity, high levels of internal social inequality, and good health access are hardest hit.
Checking COVID‐19 victims not just for underlying health conditions but also for medication histories is the only way of knowing if there might be a link to chronic multiple medication use. Much more research is needed to figure out how susceptibility to dying with COVID‐19 might be related to medication patterns. But to date, neither clinicians, epidemiologists, nor social scientists are studying this. We need to link COVID‐19 deaths to clustered disorders, and we need to link clustered disorders to clustered overmedication. The short ethnographic case study I presented here is only a tiny fragment of the work that needs to be done on experiences and causes of multimorbidity, chronic medication use, and polyiatrogenesis in a pandemic era. Both clinical and epidemiological findings are suggestive of links between relative socioeconomic deprivation, medication overuse, and susceptibility to the novel coronavirus. As long as there is no effective medication to treat COVID‐19, clinicians should make sure that existing treatments for other medical conditions are used with greater care.
References Cited
- Academy of Medical Sciences . 2018. Multimorbidity: A Priority for Global Health Research , p. 5. https://acmedsci.ac.uk/policy/policy-projects/multimorbidity-helpful-resources (accessed October 26, 2020).
- Aiden, H. 2018. Multimorbidity: Understanding the Challenge. A Report for the Richmond Group of Charities . https://richmondgroupofcharities.org.uk/sites/default/files/multimorbidity_-_understanding_the_challenge.pdf (accessed Oct. 26, 2020)
- American Heart Association (AHA) . 2020. Coronavirus Questions for Patients. https://www.heart.org/en/coronavirus/coronavirus-questions/if-youre-a-patient (accessed May 12, 2020).
- Barnett, K. , Mercer S. W., Norbury M., Watt G., Wyke S., and Guthrie B.. 2012. Epidemiology of Multimorbidity and Implications for Health Care, Research, and Medical Education: A Cross‐sectional Study. The Lancet 380: 37–43. [DOI] [PubMed] [Google Scholar]
- Béhague, D. P. 2015. “Taking Pills for Developmental Ails in Southern Brazil: The Biologization of Adolescence?” Social Science & Medicine 143: 320–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biehl, J. 2005. Vita: Life in a Zone of Social Abandonment. Berkeley: University of California Press. [Google Scholar]
- Busija, L. , Lim K., Szoeke C., Sanders K. M., and McCabe M. P.. 2019. Do Replicable Profiles of Multimorbidity Exist? Systematic Review and Synthesis. European Journal of Epidemiology 34: 1025–53. [DOI] [PubMed] [Google Scholar]
- Clark, H. , and Whiteley P.. 2020. Economic Inequality Can Help Predict COVID‐19 Deaths in the US. London School of Economics US Center Blog. https://blogs.lse.ac.uk/usappblog/2020/05/06/economic-inequality-can-help-predict-covid-19-deaths-in-the-us/ (accessed Oct. 26, 2020).
- Day, M. 2020. Covid‐19: Four‐fifths of Cases Are Asymptomatic, China Figures Indicate. British Medical Journal 369: m1375. [DOI] [PubMed] [Google Scholar]
- Docherty, A. B. , Harrison E. M., Green C. A., Hardwick H. E., Pius R., Norman L., Holden K. A., Read J. M., Dondelinger F., Carson G., Merson L., Lee J., Plotkin D., Sigfrid L., Halpin S., Jackson C., Gamble C., Horby P. W., Nguyen‐Van‐Tam J. S., Dunning J., Openshaw P. J. M., Baillie J. K., Semple M. G.. 2020. Features of 16,749 hospitalised UK patients with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol. medRxiv 2020.04.23.20076042; 10.1101/2020.04.23.20076042 [DOI] [PMC free article] [PubMed]
- Dugravot, A. , Fayosse A., Dumurgier J., Bouillon K., Tesnim B., Rayana B., Schnitzler A., Kivimaki M., Sabia S., and Singh‐Manoux A.. 2020. Social Inequalities in Multimorbidity, Frailty, Disability, and Transitions to Mortality: A 24‐year Follow‐up of the Whitehall II Cohort Study. The Lancet Public Health 5: e42–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumit, J. 2012. Drugs for Life: How Pharmaceutical Companies Define Our Health. Durham: Duke University Press. [Google Scholar]
- Ecks, S. In press. Living Worth: Value and Values in Global Pharmaceutical Markets. Durham: Duke University Press. [Google Scholar]
- EuroHealthNet . 2020. What COVID‐19 is teaching us about inequality and the sustainability of our health systems. https://eurohealthnet.eu/COVID-19 (accessed October 26, 2020).
- EuroMOMO . 2020. European Mortality Monitoring Project: Graphs and Maps. https://www.euromomo.eu/graphs-and-maps (accessed October 26, 2020).
- Farmer, P. 1996. On Suffering and Structural Violence: A View from Below. Daedalus 125: 261–83. [Google Scholar]
- Garin, A. , Koyanagi A., Chatterji S., Tyrovolas S., Olaya B., Leonardi M., Lara E., Koskinen S., Tobiasz‐Adamczyk B., Luis Ayuso‐Mateos J., and Haro J. M.. 2016. Global Multimorbidity Patterns: A Cross‐sectional, Population‐based, Multi‐country Study. Journal of Gerontology Series A Biological Sciences and Medical Sciences 71: 205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie, B. , Payne K., Alderson P., McMurdo M. E. T., and Mercer S. W.. 2012. Adapting Clinical Guidelines to Take Account of Multimorbidity. BMJ 345: e6341. [DOI] [PubMed] [Google Scholar]
- Hanlon, P. , Nicholl B., Jani B. D., Lee D., McQueenie R., and Mair F. S.. 2018. Frailty and Pre‐frailty in Middle‐aged and Older Adults and Its Association with Multimorbidity and Mortality: A Prospective Analysis of 493 737 UK Biobank Participants. Lancet Public Health 3: e323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton, R. 2020. Offline: COVID‐19 and the NHS: A National Scandal. The Lancet 395: P1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JN Learning . 2020. COVID‐19 Update with NIAID's Anthony Fauci, MD; March 18, 2020. https://edhub.ama-assn.org/jn-learning/audio-player/18324686#section-transcript (accessed October 26, 2020).
- Kaufman, S. R. 2015. Ordinary Medicine: Extraordinary Treatments, Longer Lives, and Where to Draw the Line. Durham: Duke University Press. [Google Scholar]
- Kleinman, A. , Das V., Lock M., and Lock M. M., eds. 1997. Social Suffering. Berkeley: University of California Press. [Google Scholar]
- Lawson, K. D. , Mercer S. W., Wyke S., Grieve E., Guthrie B., G. C. M., Watt, E. and Fenwick A. E.. 2013. Double Trouble: The Impact of Multimorbidity and Deprivation on Preference‐weighted Health Related Quality of Life: A Cross‐sectional Analysis of the Scottish Health Survey. International Journal for Equity in Health 12: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock, M. , and Nguyen V. K.. 2018. An Anthropology of Biomedicine. Oxford: John Wiley & Sons. [Google Scholar]
- Manderson, L. , and Wahlberg A.. 2020. Chronic Living in a Communicable World. Medical Anthropology. Preprint. 10.1080/01459740.2020.1761352 [DOI] [PubMed] [Google Scholar]
- Mangin, D. , and Garfinkel D.. 2019. Foreword to the First Special Collection: Addressing the Invisible Iatrogenic Epidemic: The Role of Deprescribing in Polypharmacy and Inappropriate Medication Use. Therapeutic Advances in Drug Safety 10: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini, J. J. , and Gattinoni L.. 2020. Management of COVID‐19 Respiratory Distress. JAMA Insights , April 24. 10.1001/jama.2020.6825 (accessed October 26, 2020). [DOI] [PubMed]
- McQueenie, R. , Foster H., Jani B. D., Katikireddi S. V., Sattar N., Pell J. P., Ho F. K., Niedzwiedz C. L., Hastie C. E., Anderson J., and Mark P. B.. 2020. Multimorbidity, Polypharmacy, and COVID‐19 Infection within the UK Biobank Cohort. medRxiv [Preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendenhall, E. 2012. Syndemic Suffering: Social Distress, Depression, and Diabetes among Mexican Immigrant Women. Walnut Creek, CA: Left Coast Press, Inc. [Google Scholar]
- Mendenhall, E. 2016. Beyond Comorbidity: A Critical Perspective of Syndemic Depression and Diabetes in Cross‐cultural Contexts. Medical Anthropology Quarterly 30: 462–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New Statesman . 2020. People from Scotland's Most Deprived Areas Twice as Likely to Die from Covid‐19 than Those in Wealthiest Areas. https://www.newstatesman.com/2020/05/people-scotlands-most-deprived-areas-twice-likely-die-covid-19-those-wealthiest-areas (accessed October 26, 2020).
- Novel Coronavirus Pneumonia Emergency Response Epidemiology Team . 2020. The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID‐19) in China. Chinese Journal of Epidemiology 41: Epub ahead of print. 10.3760/cma.j.issn.0254-6450.2020.02.003 (accessed October 26, 2020). [DOI] [Google Scholar]
- Ouyang, H. 2020. I'm an E.R. Doctor in New York. None of Us Will Ever Be the Same. The New York Times Magazine, April 14.
- O'Brien, R. , Wyke S., Guthrie B., Watt G., and Mercer S.. 2011. An “Endless Struggle”: A Qualitative Study of General Practitioners’ and Practice Nurses’ Experiences of Managing Multimorbidity in Socio‐economically Deprived Areas of Scotland. Chronic Illness 7: 45–59. [DOI] [PubMed] [Google Scholar]
- Our World in Data . 2020. Statistics and Research: Coronavirus Pandemic https://ourworldindata.org/coronavirus#all-charts-preview (accessed August 13, 2020).
- Patel, A. B. , and Verma A.. 2020. COVID‐19 and Angiotensin‐converting Enzyme Inhibitors and Angiotensin Receptor Blockers: What Is the Evidence? JAMA March 24. https://jamanetwork.com/journals/jama/article-abstract/2763803 (accessed October 26, 2020). [DOI] [PubMed]
- Payne, R. A. , Mendonca S. C., Elliott M. N., Saunders C. L., Edwards D. A., Marshall M., and Roland M.. 2020. Development and Validation of the Cambridge Multimorbidity Score. Canadian Medical Association Journal 192: E107–E114; 10.1503/cmaj.190757 (accessed October 26, 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulla, P. 2020. “The Epidemic Is Growing Very Rapidly”: Indian Government Adviser Fears Coronavirus Crisis Will Worsen. Nature, June 26. https://www.nature.com/articles/d41586-020-01865-w?fbclid=IwAR3g4Pbyp3N8sMzfXUBnK5IvOB5ftHydR8X2-9SADvwWwKgz1Xi57__vF9s (accessed October 26, 2020). [DOI] [PubMed]
- Richardson S., Hirsch J. S., and Narasimhan M.. 2020. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized with COVID‐19 in the New York City Area. JAMA. https://jamanetwork.com/journals/jama/fullarticle/2765184 (accessed April 22, 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, M. , Levi M., Schilling R., Lim W. S., Grocott M. P. W., and McKee M.. 2020. Covid‐19: A Complex Multisystem Clinical Syndrome. BMJ Opinion, May 1. https://blogs.bmj.com/bmj/2020/05/01/covid-19-a-complex-multisystem-clinical-syndrome/ (accessed October 26, 2020).
- Scheper‐Hughes, N. 1992. Death without Weeping: The Violence of Everyday Life in Brazil. Berkeley: University of California Press. [Google Scholar]
- Schiøtz, M. L. , Høst D., and Frølich A.. 2016. Involving Patients with Multimorbidity in Service Planning: Perspectives on Continuity and Care Coordination. Journal of Comorbidity 6: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer, M. , Bulled N., Ostrach B., and Mendenhall E.. 2017. Syndemics and the Biosocial Conception of Health. The Lancet 389: 941–50. [DOI] [PubMed] [Google Scholar]
- Stineman, M.G. , Xie D., Pan Q., Kurichi J. E., Zhang Z., Saliba D., Henry‐Sánchez J. T., and Streim J.. 2012. All‐cause 1‐, 5‐, and 10‐year Mortality in Elderly People According to Activities of Daily Living Stage. Journal of the American Geriatrics Society 60: 485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Aa, M. J. , van den Broeke J. R., Stronks K., and Plochg T.. 2017. Patients with Multimorbidity and Their Experiences with the Healthcare Process: A Scoping Review. Journal of Comorbidity 7: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle, S. 2019. Ten‐minute Appointments Too Short to Be Useful and Must Be Phased out by 2030, Say GPs. The Independent, May 21. https://www.independent.co.uk/news/health/gp-appointments-ten-minutes-phase-out-2030-royal-college-nhs-a8922106.html (accessed October 26, 2020).
- Weaver, L. J. , Barrett R., and Nichter M.. 2016. Special Section on Comorbidity: Introduction. Medical Anthropology Quarterly 30: 435–41. [DOI] [PubMed] [Google Scholar]
- White, K. 2006. The Sage Dictionary of Health and Society. London: Sage. [Google Scholar]
- Whitty, C. J. M. , MacEwen C., Goddard A., Alderson D., Marshall M., Calderwood C., Atherton F., McBride M., Atherton J., Stokes‐Lampard H., Reid W., Powis S., and Marx C.. 2020. Editorial: Rising to the Challenge of Multimorbidity. The British Medical Journal 368: l6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann, D. , Sperhake J., Lütgehetmann M., Steurer S., Edler C., Heinemann A., Heinrich F., Mushumba H., Kniep I., Schröder A. S., Burdelski C., de Heer G., Nierhaus A., Frings D., Pfefferle S., Becker H., Bredereke‐Wiedling H., de Weerth A., Paschen H., Sheikhzadeh‐Eggers S., Stang A., Schmiedel S., Bokemeyer C., Addo M. M., Aepfelbacher M., Püschel K., and Kluge S.. 2020. Autopsy Findings and venous Thromboembolism in Patients with COVID‐19: A Prospective Cohort Study. Annals ofInternal Medicine, May 6. 10.7326/M20-2003 (accessed October 26, 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunder, O. 2020. Rechtsmediziner: “Ohne Vorerkrankung ist in Hamburg an Covid‐19 noch keiner gestorben.” Hamburger Morgenpost, May 6.


