Abstract
Human induced Pluripotent Stem Cell (hiPSC) models are a valuable new tool for research into neurodegenerative diseases. Neuroinflammation is now recognized as a key process in neurodegenerative disease and aging, and microglia are central players in this. A plethora of hiPSC-derived microglial models have been published recently to explore neuroinflammation, ranging from monoculture through to xenotransplantation. However, combining physiological relevance, reproducibility, and scalability into one model is still a challenge. We examine key features of the in vitro microglial environment, especially media composition, extracellular matrix, and co-culture, to identify areas for improvement in current hiPSC-microglia models.
Keywords: microglia, human, induced pluripotent stem cells, in vitro models, media composition, 3D scaffolds, co-culture, physiological relevance
Introduction
Microglia represent a branch of tissue-resident macrophages which originate primarily in the yolk sac (1) and finally mature in the central nervous system (CNS) (2, 3). Once within the CNS, microglia function as important homeostatic cells and innate immune sentinels, attracting the analogy of a double-edged sword through their contribution to both health and disease. They influence developing neural networks by regulating the rate of neurogenesis and pruning neuronal synapses [(4), reviewed by (5)]. As ramified cells, they aid brain homeostasis by surveying the brain parenchyma and clearing cellular debris. However, as amoeboid cells, they secrete cytokines in response to pathogens (e.g. viral infection), misfolded proteins (e.g. Alzheimer’s disease), or cellular damage (e.g. brain injury). The cytokine response can exacerbate neuronal damage if prolonged, contributing to chronic neurodegenerative disease [(6), reviewed by (7)]. This key pathological process is still inadequately understood, hindered by the lack of good quality in vitro models.
Why Use hiPSC-Microglia to Model Microglia?
Human induced Pluripotent Stem Cells (hiPSC) offer unprecedented advantages over other commonly used primary and immortalized cell cultures, notably human genetic background, normal karyotype, limitless self-renewal, and suitability for gene editing [reviewed by (8)]. The human genetic background solves the poor representation of disease-relevant gene orthologs in mouse models (9, 10) deemed partly responsible for the poor success-rate of translating therapeutic modalities from mouse studies into the clinic [reviewed by (11, 12)]. Many neurological diseases are multigenic, and therefore particularly difficult to model in mice, whereas hiPSC enable representation of extremes of polygenic risk score (13), and endogenous levels of protein expression.
Microglial identity is driven by both developmental origin and brain environment [reviewed by (14)]. They derive from early waves of primitive yolk sac macrophages, which migrate to the brain rudiment even before neural progenitors develop (1, 15), and are maintained by local self-renewal throughout life (16). Several strategies have been published recently to derive microglia from hiPSC, which generally mirror the main characteristics of primitive hematopoiesis, including dependence on PU.1 and IRF8 transcription factors and MYB independence [(15, 17) reviewed by (18)], though the in vitro developmental pathway does not necessarily conform exactly to in vivo primitive waves. While mesoderm/hemogenic endothelium/myeloid differentiation can be achieved using simple growth factor cocktails (minimally BMP4, VEGF, SCF, followed by IL-3, M-CSF), mature microglial identity subsequently relies on cues present in the CNS environment, and this is where protocols differ in terms of their final medium composition and use of monoculture versus co-culture to induce microglial maturation (19–28). Some protocols have been more widely adopted and implemented within independent labs and used at scale by companies, making them highly reproducible [see (19, 26, 29) and (25) adopted by (27, 30)].
Within hours of isolation from the brain, both human and rodent primary microglia downregulate several key mature microglial markers, particularly TMEM119, P2RY12, and SALL1 (2, 31), which can be restored upon re-transplantation into the brains of microglia-deficient mice (32). Likewise, hiPSC-microglia assume the closest identity to in vivo microglia when they are transplanted into rodent brains (9, 19, 33–35). However, this is clearly not practical for high-throughput experiments, and is still limited by the xenogenic environment. Therefore, further improving in vitro hiPSC-microglia models to better represent their in vivo counterparts remains paramount. The pros and cons of different hiPSC-microglia models, and their application for disease modeling, have been reviewed recently (18, 36–39), but how to improve the physiological relevance of these models is an under-covered area. In this mini review, we focus on how to better mimic the CNS habitat, with relevant media composition, extracellular matrix, and co-culture, which will further improve the physiological applications and reproducibility of existing in vitro hiPSC-microglia protocols.
Improving hiPSC-Microglia Media Composition
To better understand, compare, and control hiPSC-microglia, we need fully defined, open-source media, which not only supply relevant survival/differentiation factors, but also reflect the composition of the brain interstitial fluid, mimicking ionic composition, energy-substrates, nutrients, minerals, vitamins, pH, and osmolarity. However, many conventional media compositions are not at all physiological, but rather a hangover from culturing cancer cell lines with high metabolic and proliferative rates. Firstly, media with high levels of glucose (up to 25 mM, versus physiological levels of 5 mM), can mask cellular phenotypes (40, 41), which is problematic, as microglial metabolic dysregulation is associated with disease phenotype (42). Secondly, while serum is a major source of nutrients, and its use made cell-culture of microglia possible in the first place (43), it is still often used for in vitro culture of glial cells, despite the fact that glia are not exposed to serum under normal conditions in vivo, as they are separated by the blood-brain barrier [reviewed by (44)]. Further, each serum batch is different, its composition is undefined, and it contains lipopolysaccharide (LPS)-binding proteins which aid binding to toll-like receptor 4 (TLR4), thereby enhancing microglial responses to LPS (45). Thirdly, a hangover from explant cultures are components which contain immunosuppressive molecules, notably B27 supplement, developed to prevent excessive cell death and reactivity, but which interfere with microglia responses (46).
Defined, more physiological media now exist [e.g. BrainPhys (47)], and serum-free conditions have been demonstrated to be viable for rodent microglia with the recently developed “TIC media.” TIC supplements DMEM/F12 with astrocyte-derived factors TGF-β, IL-34, and cholesterol (32). TGF-β promotes maturation and specialization of microglia within the developing brain, helps maintain the identity of cultured microglia [both when isolated as primary cells (2, 31) or derived from human iPSCs (19)], and encourages an anti-inflammatory quiescent microglia phenotype (48). IL-34 is the main ligand in the brain for Colony Stimulating Factor 1 receptor (CSF-1R), signaling through which is essential for microglial survival (1, 49). Cholesterol further improved the benefit conveyed by IL-34/TGF-β, despite myeloid cells being capable of de novo cholesterol synthesis [(50) reviewed by (51)]. However, despite TIC factors enabling survival, serum was still required to initiate microglial phagocytic activity (32). Overall, whilst the field is clearly moving towards defined, physiological media for hiPSC-microglia, serum confounds this, and use of immunomodulatory additives needs to be noted when comparing inflammatory responses across studies.
Improving hiPSC-Microglia Through Scaffolded 3D Extracellular Environment
Microglia in vivo are embedded in a three-dimensional network of macromolecules derived from both neuronal and glial cells, mainly glycosaminoglycans (e.g. hyaluronic acid), proteoglycans, glycoproteins, and low levels of fibrous proteins (including collagen, laminin, fibronectin, and vitronectin), creating a rather “soft,” viscoelastic brain environment of ~3 kPa (52, 53). Unfortunately, these basic features are mostly absent in conventional in vitro conditions. Mimicking this 3D extracellular environment should enable hiPSC-microglia to adopt a more authentic phenotype. This is particularly important as immune cells are especially prone to develop unwanted and inflammatory responses to a suboptimal environment, such as covalently cross-linked (purely elastic) synthetic matrices, which lack viscoelastic properties [(54), reviewed by (55)].
3D cell culture, both scaffolded and scaffold-free, is becoming more widely used, as it preserves natural cell shape, supports cell-to-cell and cell-to-matrix communication, enhances cell differentiation, and pushes gene and protein expression towards that found in vivo [reviewed by (56)]. Surprisingly, only a few groups have cultured microglial cells in a 3D scaffolded environment ( Table 1 ), and several of them primarily focus on other cell types co-cultured with microglia, including neurons (70) and glioblastoma cells (71, 72). To properly distinguish the effects attributable to 3D conditions, systematic studies using relevant 2D controls are required for appropriate comparisons to be made.
Table 1.
Key studies of microglial culture in 3D scaffolds.
| Material | Modifications | Serum | Microglia | Observations | Reference | |
|---|---|---|---|---|---|---|
| NATURAL HYDROGEL AND DERIVATIVES | Alginate | RGD sequence | √ | Fetal rat primary | 1. Amoeboid morphologies over 2 weeks | Frampton et al. (57) |
| Methacrylated hyaluronic acid | × | √ | Postnatal rat primary | 1. Heterogenous morphology (mostly amoeboid) 2. Microglia wrapped processes around other cells—perhaps phagocytosis or cell−cell interaction |
Jeffery et al. (58) | |
| Hyaluronic acid | Combined with basal lamina mixture | √ | Postnatal rat primary | 1. Microglia in 3D hyaluronic acid hydrogel mostly small with few processes 2. Microglia in 3D hyaluronic acid lamina hydrogel more dispersed and more thin processes |
Koss et al. (59) | |
| Hyaluronic acid-gelatin | Combined with Gelin-S and heparin | √ | Human fetal immortalized CHME3 | 1. Protective effect of microglia on GBM greater in the 3D model when challenged with cytotoxics 2. Microglia promoted GBM proliferation more in 3D vs 2D | Leite et al. (60) | |
| Collagen I | × | √ | Murine immortalized BV-2 | 1. 3D collagen promotes multiplanar projections 2. Increased inflammatory response following LPS stimulation versus 2D collagen coating |
Haw et al. (61) | |
| Collagen I | × | √ | Murine immortalized BV-2 | 3D collagen hydrogel vs 2D uncoated: 1. Smaller fold change in gene expression following LPS treatment 2. Smaller fold changes in ROS production following LPS treatment | Cho et al. (62) | |
| Matrigel | × | √ | Human immortalized SV40 | 1. Microglia seeded in 3D Matrigel in an angular chamber presented protrusions 2. Microglia only migrated to the central chamber with neurons and astrocytes when neural cells were overexpressing Aβ 3. Once together with neurons and astrocytes, microglia were actively in contact with neurons and astrocytes by expanding and retracting their protrusions |
Park et al. (63) | |
| SYNTHETIC HYDROGEL & OTHERS | Synthetic peptide | Fibronectin or collagen I coating | √ | Murine immortalized BV-2 | 1. Peptide hydrogel promoted ramification 2. Fibronectin or collagen addition promoted deramification |
Pöttler et al. (64) |
| Graphene foam | × | √ | Murine immortalized BV-2 | 3D graphene vs 2D graphene: 1. did not induce further microglial ramification 2. decreased NO production following LPS stimulation 3. Conditioned medium (CM) promoted greater survival of mouse primary neural stem cells 5. CM rescued LPS-induced neuroinflammation | Song et al. (65) | |
| PEG | MMP-degradable peptide cross-links and CRGDS sequence peptide | × | Derived from human ESC line H1 | 1. Microglia adopted both ramified and ameboid morphologies in 3D co-culture with other brain cell types | Schwartz et al. (66) | |
| Graphene foam | × | √ | Murine immortalized BV-2 | 1. CM from microglia in 3D graphene (vs 2D graphene) promoted neurosphere formation, facilitated mouse primary NSC migration from neurospheres, and increased single cell polarization by activating the SDF-1α/CXCR4 signaling pathway and enhanced cell adhesion on the substrate. | Jiang et al. (67) | |
| Fmoc–Phe3 peptide | × | √ | Postnatal rat primary | 1. Fmoc–Phe3 peptide hydrogel stimulated microglial proliferation and NGF secretion (due to the peptide, not 3D conditions) | Chronopoulou et al. (68) | |
| P(TMC-CL) | × | √ | Postnatal rat primary | 1. Cells cultured within a 3D P(TMC-CL) scaffold were smaller and with elongated processes compared to cells cultured on a P(TMC-CL) flat film. 2. Following exposure to myelin, only cells in 2D conditions presented abberant multinucleated phenotype |
Pires et al. (69) |
Aβ, amyloid beta; CM, conditioned media; CRGDS, peptide sequence of Cys-Arg-Gly-Asp-Ser present in fibronection; CXCR4, C-X-C chemokine receptor 4; ESC, embryonic stem cell; GBM, glioblastoma multiforme; LPS, lipopolysaccharide; MMP, matrix metalloproteinase; NGF, nerve growth factor; NO, nitric oxide; NSC, neural stem cell; PEG, polyethylene glycol; P(TMC-CL), poly(trimethylene carbonate-co-ϵ-caprolactone); RGD, tripeptide motif of Arg-Gly-Asp present in fibronectin; SDF-1α, stromal cell-derived factor 1 α.
Scaffolded 3D culture influences microglial morphology and attachment, but the outcome depends on the scaffold composition and structure. Choosing a material that optimizes microglial phenotype still remains a challenge. Hydrogels of synthetic peptide (68), collagen (61, 64), or PEG (66) enhanced ramification in both postnatal rat microglia and immortalized murine BV-2 cells. Likewise, primary rat microglia cultured within a 3D fibrous poly(trimethylene carbonate-co-ϵ-caprolactone) scaffold had smaller cells with elongated processes versus cells attached to flat solvent-cast films (69). However, this was not observed in 3D graphene scaffolds, where BV-2 cells remained mostly amoeboid (65). Some peptides, like neural cell adhesion molecule (NCAM)-derived KHIFSDDSSE, favor neuron and astrocyte attachment but not microglial attachment (73). Functionalizing hydrogels with extracellular matrix (ECM) components have provided mixed results; a basal lamina mixture prompted primary postnatal rat microglia within a glial co-culture to disperse throughout the hyaluronic acid-based scaffold (59), whereas coating a collagen hydrogel with fibronectin and laminin decreased ramification in BV-2 cells (64).
3D culture can also affect microglial behavior and interaction with other cell types. Neuronal survival in conditioned media from 3D-cultured BV-2 cells was higher than from 2D-cultured cells, and LPS-induced BV-2-mediated neuronal toxicity was also reduced as a result of 3D microglial culture (65). Meanwhile, more BV-2 cells upregulated CD40 (induced through NF-κB and STAT-1α) in response to LPS when in 3D collagen than in 2D (61), suggesting a more homogenous inflammatory response in 3D (74). Although BV-2 and other immortalized cell lines have been a valuable resource for studying microglia, it is likely that microglial responses to a 3D environment might be masked or even reversed by the aberrantly proliferative and primed state of such cells (75, 76). Nonetheless, 3D culture of microglia in specific scaffolds seems to promote a production of growth factors and an anti-inflammatory phenotype beneficial to other cell types.
Improving hiPSC-Microglia Through Co-Culture
The simplest approach to study cross-talk between microglia and neurons or astrocytes, is by transferring medium conditioned by these cells cultured separately. This provides a high level of experimental control, as the conditioned media can be analyzed independently to identify cytokines produced by stimulated microglia (77), assess the impact of individual factors on survival (32), or interrogate the process studied using drugs. However, conditioned media only allows study of uni-directional signaling. Transwells or Boyden chambers allow different cell types to exchange secreted factors bi-directionally [reviewed by (78)]. This improves physiological relevance but offers less insight into which cell type the secreted signals originated from, or control over which cells are targeted with any manipulation or drug given. Furthermore, these culture methods are also limited by the effective concentration of secreted signals, being much lower compared to local concentrations if cells were in physical contact.
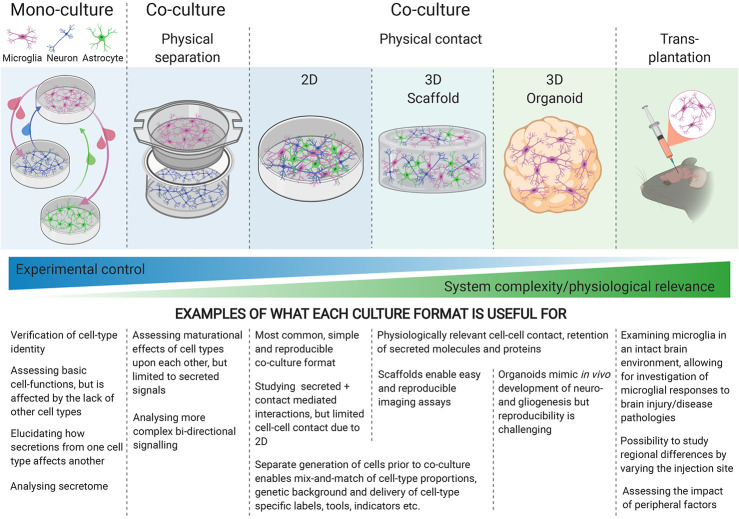
To fully capture the physiology of cellular interactions, physical contact-mediated cues, through receptor-ligand interactions, are required. Microglial quiescence is maintained through neuronal CD200 glycoprotein interaction with the microglial CD200 receptor [(79) reviewed by (80)], as well as neuronal transmembrane (but possibly also secreted) CD22 interaction with microglial CD45 receptors (81). The mainly neuronal chemokine CX3CL1 (“fractalkine”) maintains microglia in a surveying state when the membrane-bound form makes contact with the microglial CX3CR1 receptors, whereas soluble CX3CL1 has a chemoattractant effect on microglia [(82), reviewed by (83)]. Furthermore, the idea that electrical activity from neurons might play a role in immune-response suppression has been around for two decades (84), but the nature of this interaction is not well understood. Generating physical-contact co-cultures would evidently enable microglia to experience all modes of interaction with other cell types, yet when increasing the physiological relevance of the culture model, the degree of control over the experimental system decreases, highlighting the importance of choosing the appropriate culture-model for each research question ( Figure 1 ).
Figure 1.
Different culture environments convey different levels of experimental control and physiological relevance, with mono-cultures at one end of the spectrum, and transplantation into intact brains at the other. The experimental question being investigated should guide the choice of culture-system.
Most hiPSC-microglia studies have focused on characterizing the gene expression and functionality of the resulting microglia in monoculture [reviewed by (8, 36)], but a few have included co-cultures with neurons (19, 24, 27) in 2D (3D co-cultures will be discussed later). Physical contact with neurons increases the expression of mature microglial genes, enhances ramified morphology compared to conditioned media, as well as eliciting a dampened response to LPS + IFNγ, high microglial motility and extension of processes to sites of injury (19, 24, 27), reflecting the homeostatic surveillance and response to injury associated with microglia in vivo (85). The exact cues responsible for these phenotypes are yet to be elucidated. Our understanding of interactions between microglia and astrocytes is limited, these cells typically being examined in isolation, but interest in their complex relationship, in terms of maintaining homeostasis in health and reactive cross-talk in disease, is growing [reviewed by (86)]. Pandya et al. used astrocytes as a feeder layer for maturing their hiPSC-microglia for 1–2 weeks before isolating the CD39+ microglia for monoculture (23), but interactions between mature hiPSC-microglia and astrocytes are otherwise currently under-explored.
The maturity and functionality of the non-microglial co-cultured cells is important to consider, as crucial microglial functions like synaptic pruning are likely only observable if the synaptic network is sufficiently mature, which may depend on more than bi-culture. Tri-culture of microglia with neurons and astrocytes will enable further maturation, more fully capture additional aspects of cross-talk and identify potential compensatory mechanisms between different brain cell types. Microglia and astrocytes operate in concert, both possessing that “double-edged sword” capacity to either support neuronal recovery (simplistically, “helpful” M2 and A2 phenotypes) or cause neuronal death (“harmful” M1 and A1 reactive phenotypes) [(77) reviewed by [87)]. A primary rodent tri-culture system of neurons, astrocytes and microglia in serum-free conditions (using TIC-supplemented media) shows promise in modeling different neuroinflammatory scenarios (88), in line with both in vivo and in vitro studies (77, 89, 90). However, TIC factors could not be omitted in the tri-cultures (88), indicating that endogenous secretion from the cultured astrocytes was not sufficient to retain a viable and physiologically active microglial phenotype. Interestingly, co-culture with hiPSC-neurons and astrocytes is capable of shifting the transcriptional profile of hiPSC-microglia further towards an ex vivo state than TIC media, although the gap between in vitro and ex vivo microglia is not fully closed (29).
Oligodendrocytes, endothelial cells, and/or vasculature are also likely to affect microglial function, yet they are rarely included in in vitro culture models, or assessment of their impact on microglia is lacking (66). Excitingly though, with the advent of single-cell RNAseq and single-cell proteomics, analyzing complex co-culture systems has become more straightforward and informative. These high-throughput methods allow for detection of overall cellular changes and activation/inactivation of biological pathways within each cell type, providing important clues to understand inter-cellular cross-talk. Using hiPSC, it is now possible to “mix and match” the genetic background of each cell type within the co-culture, such that the effect of a mutation can be studied in one cell type at a time. Strategies for co-culturing multiple hiPSC cell-types are increasingly popular and are beginning to move towards three dimensional environments as well.
Combining Co-Culture and 3D Environment for hiSC-Microglia
Providing microglia with a more physiologically relevant cellular environment relies on the principle of letting separately derived microglia populate pre-existing 3D neuronal cultures, brain organoids, or xenotransplantation into rodent brains [reviewed by (18)]. 3D scaffolded cultures confer a high degree of reproducibility and experimental flexibility, as individual cell types can be derived separately and added to the scaffold one at a time. This enables mix and match of co-cultures, i.e. cell types, ratios, genetic backgrounds, genetic tools, and fluorescent reporters. A 3D scaffold culture with microfluidic chambers has demonstrated that immortalized human SV40-derived microglia display ramified morphologies when seeded within Matrigel, migrate towards Amyloid-beta (Aβ)-expressing neurons, and cleave their axons while producing neurotoxic mediators (63). This encouraging study could be made more physiologically relevant by replacing the genetically aberrant SV40-microglial cells with hiPSC-derived microglia, and mouse sarcoma-derived Matrigel with a defined hydrogel more reminiscent of brain ECM.
3D organoids preserve the human model context, yet high variability between organoid batches remains a technical challenge. Remarkably, cerebral organoids spontaneously containing microglia have been obtained (91), but this is only apparent when mesoderm is not sufficiently suppressed. More conventionally, organoids and microglia are derived separately, due to the differing ontogeny between ectodermally derived CNS cells and mesodermally derived microglia. Integrated microglia adopt ramified morphologies, display migration to the site of injury (19, 20, 28) and phagocytose Aβ (92).
Xenotransplantation offers the benefit of transplanting hiPSC-derived microglia into a more mature and structurally developed environment. However, immune-deficiency and expression of human CSF1 or IL34 are crucial for survival of the transplanted human microglia (33, 93), and ablation of host microglia is usually preferable. Excitingly, several recent xenotransplantation studies have detected improved hiPSC-microglial morphologies, with transcriptional profiles showing higher degrees of similarity between xenotransplanted microglia and freshly isolated human microglia than with cultured primary microglia (9, 33, 34). Meanwhile, the pattern of differentially expressed genes revealed a more muted response to LPS in transplanted cells than in in vitro cultured microglia (33). Engrafted microglia associate with Aβ plaques, appear to phagocytose them (19, 33), and upregulate disease-associated microglia markers, including CD9, MERTK, and TREM2 (33). Xenotransplantation is therefore a viable option for studying hiPSC-derived human microglia allowed to mature in an in vivo brain environment, where the issue of altered in vitro gene expression (31, 32) appears to have been resolved, but making this technique amenable to large scale-up will be extremely challenging.
Discussion
Strides towards more physiological hiPSC-microglia culture conditions are being made apace, including the use of defined serum-free media in monoculture, the implementation of co-cultures, whether in 2D format, organoids, or xenotransplantation. One important underdeveloped gap in the repertoire is an in vitro model containing multiple hiPSC types embedded in a defined 3D scaffold, which would both enhance microglial identity and be amenable to advanced approaches, including arrayed drug screening, genome-wide CRISPR screens, high content imaging, cell multiplexing, and single cell transcriptomics. Further work is necessary to identify viscoelastic scaffold materials and ECM components that optimize microglial morphology, adhesion, and function. The process of neuroinflammation is arguably best modeled with all participating cell types present. Loss of function in astrocytes and/or microglia, impacting homeostatic support, or a toxic gain of function in the same cells, leading to the release of neurotoxic factors, would both probably have detrimental effects on neuronal health. Both scenarios are likely occurring in tandem in various neurodegenerative diseases, as well as in normal ageing, where defective phagocytosis as well as pro-inflammatory profiles have recently been reported (94, 95). Many links in the harmful inflammatory cascades that have been demonstrated to originate with microglia, propagate to astrocytes, and ultimately cause neurodegeneration, are not fully elucidated [(77), reviewed by (87)], and there is much more to learn about cross-talk and compensatory mechanisms between these cell types. Ultimately, the trade-off between the physiological relevance of a culture-system and experimental control over conditions must be decided based on the scientific question asked. Various hiPSC-microglia models will continue to be relevant, but the more defined and comparable we can make them, the better we will be able to understand and control the double-edged sword that is microglial function.
Author Contributions
Conceptualization: SS, AH, and SC. Writing—Original Draft: SS and AH. Writing—Review and Editing: SS, AH, WJ, and SC. Funding Acquisition: WJ and SC. Supervision: WJ and SC. All authors contributed to the article and approved the submitted version.
Funding
We thank the following for financial support: The Oxford Martin School for core support to the James Martin Stem Cell Facility (LC0910-004; SC); BBSRC industrial CASE studentship (BB/M011224/1; SS); Eli Lilly and Company (AH, SS). The authors declare that this study received funding from Eli Lilly and Company. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The figure was created with BioRender.com.
References
- 1. Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science (80) (2010) 330:841–5. 10.1126/science.1194637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, et al. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat Neurosci (2014) 17:131–43. 10.1038/nn.3599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Swinnen N, Smolders S, Avila A, Notelaers K, Paesen R, Ameloot M, et al. Complex invasion pattern of the cerebral cortex bymicroglial cells during development of the mouse embryo. Glia (2013) 61:150–63. 10.1002/glia.22421 [DOI] [PubMed] [Google Scholar]
- 4. Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic pruning by microglia is necessary for normal brain development. Science (80) (2011) 333:1456–8. 10.1126/science.1202529 [DOI] [PubMed] [Google Scholar]
- 5. Sakai J. Core Concept: How synaptic pruning shapes neural wiring during development and, possibly, in disease. Proc Natl Acad Sci USA (2020) 117:16096–9. 10.1073/pnas.2010281117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matcovitch-Natan O, Winter DR, Giladi A, Aguilar SV, Spinrad A, Sarrazin S, et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science (2016) 353(6301):aad8670. 10.1126/science.aad8670 [DOI] [PubMed] [Google Scholar]
- 7. Li Q, Barres BA. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol (2018) 18:225–42. 10.1038/nri.2017.125 [DOI] [PubMed] [Google Scholar]
- 8. Sabogal-Guáqueta AM, Marmolejo-Garza A, de Pádua VP, Eggen B, Boddeke E, Dolga AM. Microglia alterations in neurodegenerative diseases and their modeling with human induced pluripotent stem cell and other platforms. Prog Neurobiol (2020) 190:101805. 10.1016/j.pneurobio.2020.101805 [DOI] [PubMed] [Google Scholar]
- 9. Mancuso R, Van Den Daele J, Fattorelli N, Wolfs L, Balusu S, Burton O, et al. Stem-cell-derived human microglia transplanted in mouse brain to study human disease. Nat Neurosci (2019) 22:2111–6. 10.1038/s41593-019-0525-x [DOI] [PubMed] [Google Scholar]
- 10. Dubbelaar ML, Kracht L, Eggen BJL, Boddeke EWGM. The Kaleidoscope of Microglial Phenotypes. Front Immunol (2018) 9:1753. 10.3389/fimmu.2018.01753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sayed N, Liu C, Wu JC. Translation of Human-Induced Pluripotent Stem Cells from Clinical Trial in a Dish to Precision Medicine. J Am Coll Cardiol (2016) 67:2161–76. 10.1016/j.jacc.2016.01.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dawson TM, Golde TE, Lagier-Tourenne C. Animal models of neurodegenerative diseases. Nat Neurosci (2018) 21:1370–9. 10.1038/s41593-018-0236-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rehbach K, Zhang H, Das D, Abdollahi S, Prorok T, Ghosh S, et al. Publicly available hiPSC lines with extreme polygenic risk scores for modeling schizophrenia. bioRxiv (2020). 10.1101/2020.07.04.185348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bennett ML, Bennett FC. The influence of environment and origin on brain resident macrophages and implications for therapy. Nat Neurosci (2020) 23:157–66. 10.1038/s41593-019-0545-6 [DOI] [PubMed] [Google Scholar]
- 15. Bian Z, Gong Y, Huang T, Lee CZW, Bian L, Bai Z, et al. Deciphering human macrophage development at single-cell resolution. Nature (2020) 582:571–6. 10.1038/s41586-020-2316-7 [DOI] [PubMed] [Google Scholar]
- 16. Askew K, Li K, Olmos-Alonso A, Garcia-Moreno F, Liang Y, Richardson P, et al. Coupled Proliferation and Apoptosis Maintain the Rapid Turnover of Microglia in the Adult Brain. Cell Rep (2017) 18:391–405. 10.1016/j.celrep.2016.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buchrieser J, James W, Moore MD. Human Induced Pluripotent Stem Cell-Derived Macrophages Share Ontogeny with MYB-Independent Tissue-Resident Macrophages. Stem Cell Rep (2017) 8:334–45. 10.1016/j.stemcr.2016.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hasselmann J, Blurton-Jones M. Human iPSC-derived microglia: A growing toolset to study the brain’s innate immune cells. Glia (2020) 68:721–39. 10.1002/glia.23781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abud EM, Ramirez RN, Martinez ES, Healy LM, Cecilia HH, Newman SA, et al. iPSC-derived human microglia-like cells to study neurological diseases. Neuron (2017) 94:278–93. 10.1016/j.neuron.2017.03.042.iPSC-derived [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brownjohn PW, Smith J, Solanki R, Lohmann E, Houlden H, Hardy J, et al. Functional Studies of Missense TREM2 Mutations in Human Stem Cell-Derived Microglia. Stem Cell Rep (2018) 10:1294–307. 10.1016/j.stemcr.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Claes C, Van Den Daele J, Boon R, Schouteden S, Colombo A, Monasor LS, et al. Human stem cell–derived monocytes and microglia-like cells reveal impaired amyloid plaque clearance upon heterozygous or homozygous loss of TREM2. Alzheimer’s Dement (2019) 15:453–64. 10.1016/j.jalz.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 22. Douvaras P, Sun B, Wang M, Kruglikov I, Lallos G, Zimmer M, et al. Directed Differentiation of Human Pluripotent Stem Cells to Microglia. Stem Cell Rep (2017) 8:1516–24. 10.1016/j.stemcr.2017.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pandya H, Shen MJ, Ichikawa DM, Sedlock AB, Choi Y, Johnson KR, et al. Differentiation of human and murine induced pluripotent stem cells to microglia-like cells. Nat Neurosci (2017) 20:753–9. 10.1038/nn.4534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takata K, Kozaki T, Lee CZW, Thion MS, Otsuka M, Lim S, et al. Induced-Pluripotent-Stem-Cell-Derived Primitive Macrophages Provide a Platform for Modeling Tissue-Resident Macrophage Differentiation and Function. Immunity (2017) 47:183–98.e6. 10.1016/j.immuni.2017.06.017 [DOI] [PubMed] [Google Scholar]
- 25. Garcia-reitboeck P, Phillips A, Piers TM, Houlden H, Hardy J, Pocock JM, et al. Human Induced Pluripotent Stem Cell-Derived Mutations Show Specific Deficits in Phagocytosis Article Human Induced Pluripotent Stem Cell-Derived Microglia-Like Cells Harboring TREM2 Missense Mutations Show Specific Deficits in Phagocytosis. CellReports (2018) 24:2300–11. 10.1016/j.celrep.2018.07.094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McQuade A, Coburn M, Tu CH, Hasselmann J, Davtyan H, Blurton-Jones M. Development and validation of a simplified method to generate human microglia from pluripotent stem cells. Mol Neurodegener (2018) 13:1–13. 10.1186/s13024-018-0297-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haenseler W, Sansom SN, Buchrieser J, Newey SE, Moore CS, Nicholls FJ, et al. A Highly Efficient Human Pluripotent Stem Cell Microglia Model Displays a Neuronal-Co-culture-Specific Expression Profile and Inflammatory Response. Stem Cell Rep (2017) 8:1727–42. 10.1016/j.stemcr.2017.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Muffat J, Li Y, Yuan B, Mitalipova M, Omer A, Corcoran S, et al. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat Med (2016) 22:1358–67. 10.1038/nm.4189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grubman A, Vandekolk TH, Schröder J, Sun G, Hatwell-Humble J, Chan J, et al. A CX3CR1 Reporter hESC Line Facilitates Integrative Analysis of In-Vitro-Derived Microglia and Improved Microglia Identity upon Neuron-Glia Co-culture. Stem Cell Rep (2020) 14:1018–32. 10.1016/j.stemcr.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gutbier S, Wanke F, Dahm N, Rümmelin A, Zimmermann S, Christensen K, et al. Large-scale production of human IPSC-derived macrophages for drug screening. Int J Mol Sci (2020) 21:1–23. 10.3390/ijms21134808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gosselin D, Skola D, Coufal NG, Holtman IR, Schlachetzki JCM, Sajti E, et al. An environment-dependent transcriptional network specifies human microglia identity. Science (80) (2017) 356:1248–59. 10.1126/science.aal3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bohlen CJ, Bennett FC, Tucker AF, Collins HY, Mulinyawe SB, Barres BA. Diverse requirements for microglial survival, specification, and function revealed by defined-medium cultures. Neuron (2017) 94:759–73. 10.1016/j.neuron.2017.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hasselmann J, Coburn MA, England W, Figueroa Velez DX, Kiani Shabestari S, Tu CH, et al. Development of a Chimeric Model to Study and Manipulate Human Microglia In Vivo. Neuron (2019) 103:1016–33.e10. 10.1016/j.neuron.2019.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Svoboda DS, Barrasa MI, Shu J, Rietjens R, Zhang S, Mitalipova M, et al. Human iPSC-derived microglia assume a primary microglia-like state after transplantation into the neonatal mouse brain. Proc Natl Acad Sci USA (2019) 116:25293–303. 10.1073/pnas.1913541116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu R, Li X, Boreland AJ, Posyton A, Kwan K, Hart RP, et al. Human iPSC-derived mature microglia retain their identity and functionally integrate in the chimeric mouse brain. Nat Commun (2020) 11:1–16. 10.1038/s41467-020-15411-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Haenseler W, Rajendran L. Concise Review: Modeling Neurodegenerative Diseases with Human Pluripotent Stem Cell-Derived Microglia. Stem Cells (2019) 37:724–30. 10.1002/stem.2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miron VE, Priller J. Investigating Microglia in Health and Disease : Challenges and Opportunities. Trends Immunol (2020) 41(9):785–93. 10.1016/j.it.2020.07.002 [DOI] [PubMed] [Google Scholar]
- 38. Speicher AM, Wiendl H, Meuth SG, Pawlowski M. Generating microglia from human pluripotent stem cells: Novel in vitro models for the study of neurodegeneration. Mol Neurodegener (2019) 14:1–16. 10.1186/s13024-019-0347-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hanger B, Couch A, Rajendran L, Srivastava DP, Vernon AC. Emerging Developments in Human Induced Pluripotent Stem Cell-Derived Microglia: Implications for Modelling Psychiatric Disorders With a Neurodevelopmental Origin. Front Psychiatry (2020) 11:789. 10.3389/fpsyt.2020.00789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kleman AM, Yuan JY, Aja S, Ronnett GV, Landree LE. Physiological glucose is critical for optimized neuronal viability and AMPK responsiveness in vitro . J Neurosci Methods (2008) 167:292–301. 10.1016/j.jneumeth.2007.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rocktäschel P, Sen A, Cader MZ. High glucose concentrations mask cellular phenotypes in a stem cell model of tuberous sclerosis complex. Epilepsy Behav (2019) 101:106581. 10.1016/j.yebeh.2019.106581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Andreone BJ, Przybyla L, Llapashtica C, Rana A, Davis SS, van Lengerich B, et al. Alzheimer’s-associated PLCγ2 is a signaling node required for both TREM2 function and the inflammatory response in human microglia. Nat Neurosci (2020) 23:927–38. 10.1038/s41593-020-0650-6 [DOI] [PubMed] [Google Scholar]
- 43. Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci (1986) 6:2163–78. 10.1523/jneurosci.06-08-02163.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Witting A, Möller T. Microglia Cell Culture: A Primer for the Novice. In: In Vitro Neurotoxicology. Totowa, NJ: Springer Protocols by Humana Press; (2011). p. 49–66. 10.1007/978-1-61779-170-3 [DOI] [PubMed] [Google Scholar]
- 45. Schumann RR, Leong SR, Flaggs GW, Gray PW, Wright SD, Mathison JC, et al. Structure and Function of Lipopolysaccharide Binding Protein. Science (80) (1990) 249:1429–32. 10.1126/science.2402637 [DOI] [PubMed] [Google Scholar]
- 46. Liu JJ, Mustafa S, Barratt DT, Hutchinson MR. Corticosterone preexposure increases NF-κB translocation and sensitizes IL-1β responses in BV2 microglia-like cells. Front Immunol (2018) 9:3. 10.3389/fimmu.2018.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bardy C, van den Hurk M, Eames T, Marchand C, Hernandez RV, Kellogg M, et al. Neuronal medium that supports basic synaptic functions and activity of human neurons in vitro . Proc Natl Acad Sci USA (2015) 112(25):2725–34. 10.1073/pnas.1504393112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Abutbul S, Shapiro J, Szaingurten-Solodkin I, Levy N, Carmy Y, Baron R, et al. TGF-β signaling through SMAD2/3 induces the quiescent microglial phenotype within the CNS environment. Glia (2012) 60:1160–71. 10.1002/glia.22343 [DOI] [PubMed] [Google Scholar]
- 49. Elmore MRP, Najafi AR, Koike MA, Nazih N, Spangenberg EE, Rice RA, et al. CSF1 receptor signaling is necessary for microglia viability, which unmasks a cell that rapidly repopulates the microglia- depleted adult brain. Neuron (2014) 82:380–97. 10.1016/j.neuron.2014.02.040.CSF1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nieweg K, Schaller H, Pfrieger FW. Marked differences in cholesterol synthesis between neurons and glial cells from postnatal rats. J Neurochem (2009) 109:125–34. 10.1111/j.1471-4159.2009.05917.x [DOI] [PubMed] [Google Scholar]
- 51. Loving BA, Bruce KD. Lipid and Lipoprotein Metabolism in Microglia. Front Physiol (2020) 11:393. 10.3389/fphys.2020.00393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McIlvain G, Schwarb H, Cohen NJ, Telzer EH, Johnson CL. Mechanical properties of the in vivo adolescent human brain. Dev Cognit Neurosci (2018) 34:27–33. 10.1016/j.dcn.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lam D, Enright HA, Cadena J, Peters SKG, Sales AP, Osburn JJ, et al. Tissue-specific extracellular matrix accelerates the formation of neural networks and communities in a neuron-glia co-culture on a multi-electrode array. Sci Rep (2019) 9:1–15. 10.1038/s41598-019-40128-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sadtler K, Wolf MT, Ganguly S, Moad CA, Chung L, Majumdar S, et al. Divergent immune responses to synthetic and biological scaffolds. Biomaterials (2019) 192:405–15. 10.1016/j.biomaterials.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 55. Chaudhuri O, Cooper-white J, Janmey PA, Mooney DJ, Shenoy VB. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature (2020) 584:535–46. 10.1038/s41586-020-2612-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jensen C, Teng Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front Mol Biosci (2020) 7:33. 10.3389/fmolb.2020.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Frampton JP, Hynd MR, Shuler ML, Shain W. Fabrication and optimization of alginate hydrogel constructs for use in 3D neural cell culture. Biomed Mater (2011) 6(1):015002. 10.1088/1748-6041/6/1/015002 [DOI] [PubMed] [Google Scholar]
- 58. Jeffery AF, Churchward MA, Mushahwar VK, Todd KG, Elias AL. Hyaluronic acid-based 3D culture model for in vitro testing of electrode biocompatibility. Biomacromolecules (2014) 15(6):2157–65. 10.1021/bm500318d [DOI] [PubMed] [Google Scholar]
- 59. Koss KM, Churchward MA, Jeffery AF, Mushahwar VK, Elias AL, Todd KG. Improved 3D hydrogel cultures of primary glial cells for in vitro modelling of neuroinflammation. J Vis Exp (2017) 2017:e56615. 10.3791/56615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Leite DM, Baskovic BZ, Civita P, Neto C, Gumbleton M, Pilkington GJ. A human co-culture cell model incorporating microglia supports glioblastoma growth and migration, and confers resistance to cytotoxics. FASEB J (2020) 34(1):1710–27. 10.1096/fj.201901858RR [DOI] [PubMed] [Google Scholar]
- 61. Haw RTY, Tong CK, Yew A, Lee HC, Phillips JB, Vidyadaran S. A three-dimensional collagen construct to model lipopolysaccharide-induced activation of BV2 microglia. J Neuroinflammation (2014) 11:134. 10.1186/1742-2094-11-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cho HJ, Verbridge SS, Davalos RV, Lee YW. Development of an in vitro 3D brain tissue model mimicking in vivo-like pro-inflammatory and pro-oxidative responses. Ann Biomed Eng (2017) 46:877–87. 10.1007/s10439-018-2004-z [DOI] [PubMed] [Google Scholar]
- 63. Park J, Wetzel I, Marriott I, Dréau D, D’Avanzo C, Kim DY, et al. Cho H. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nat Neurosci (2018) 21:941–51. 10.1038/s41593-018-0175-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pöttler M, Zierler S, Kerschbaum HH. An artificial three-dimensional matrix promotes ramification in the microglial cell-line, BV-2. Neurosci Lett (2006) 410:137–40. 10.1016/j.neulet.2006.09.082 [DOI] [PubMed] [Google Scholar]
- 65. Song Q, Jiang Z, Li N, Liu P, Liu L, Tang M, et al. Anti-inflammatory effects of three-dimensional graphene foams cultured with microglial cells. Biomaterials (2014) 35:6930–40. 10.1016/j.biomaterials.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 66. Schwartz MP, Hou Z, Propson NE, Zhang J, Engstrom CJ, Costa VS, et al. Human pluripotent stem cell-derived neural constructs for predicting neural toxicity. Proc Natl Acad Sci USA (2015) 112:12516–21. 10.1073/pnas.1516645112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jiang Z, Song Q, Tang M, Yang L, Cheng Y, Zhang M, et al. Enhanced migration of neural stem cells by microglia grown on a three- dimensional graphene scaffold ACS. Appl. Mater. Interfaces (2016) 8(38):25069–77. 10.1021/acsami.6b06780 [DOI] [PubMed] [Google Scholar]
- 68. Chronopoulou L, Togna AR, Guarguaglini G, Masci G, Giammaruco F, Togna GI, et al. Self-assembling peptide hydrogels promote microglial cells proliferation and NGF production. Soft Matter (2012) 8:5784–90. 10.1039/c2sm25528f [DOI] [Google Scholar]
- 69. Pires LR, Rocha DN, Ambrosio L, Pêgo AP. The role of the surface on microglia function: Implications for central nervous system tissue engineering. J R Soc Interface (2015) 12:1–10. 10.1098/rsif.2014.1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liu L, Koo Y, Akwitti C, Russell T, Gay E, Laskowitz DT, et al. Three-dimensional (3D) brain microphysiological system for organophosphates and neurochemical agent toxicity screening. PLoS One (2019) 14:e0224657. 10.1371/journal.pone.0224657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Blazquez R, Pukrop T. 3D coculture model of the brain parenchyma–metastasis interface of brain metastasis. In: Methods in Molecular Biology. New York, NY: Springer Protocols by Humana Press Inc; (2017). p. 213–22. 10.1007/978-1-4939-7021-6_16 [DOI] [PubMed] [Google Scholar]
- 72. Hermida MA, Kumar JD, Schwarz D, Laverty KG, Di Bartolo A, Ardron M, et al. Three dimensional in vitro models of cancer: Bioprinting multilineage glioblastoma models. Adv Biol Regul (2020) 75:100658. 10.1016/j.jbior.2019.100658 [DOI] [PubMed] [Google Scholar]
- 73. Sridar S, Churchward MA, Mushahwar VK, Todd KG, Elias AL. Peptide modification of polyimide-insulated microwires: Towards improved biocompatibility through reduced glial scarring. Acta Biomater (2017) 60:154–66. 10.1016/j.actbio.2017.07.026 [DOI] [PubMed] [Google Scholar]
- 74. Qin H, Wilson CA, Sun JL, Zhao X, Benveniste EN. LPS induces CD40 gene expression through the activation of NF-κB and STAT-1α in macrophages and microglia. Blood (2005) 106:3114–22. 10.1182/blood-2005-02-0759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol (1990) 27:229–37. 10.1016/0165-5728(90)90073-V [DOI] [PubMed] [Google Scholar]
- 76. Amadio S, De Ninno A, Montilli C, Businaro L, Gerardino A, Volonté C. Plasticity of primary microglia on micropatterned geometries and spontaneous long-distance migration in microfluidic channels. BMC Neurosci (2013) 141–12. 10.1186/1471-2202-14-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature (2017) 541:481–7. 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Guttenplan KA, Liddelow SA. Astrocytes and microglia: Models and tools. J Exp Med (2019) 216:71–83. 10.1084/jem.20180200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wright GJ, Puklavec MJ, Willis AC, Hoek RM, Sedgwick JD, Brown MH, et al. Lymphoid/neuronal cell surface OX2 glycoprotein recognizes a novel receptor on macrophages implicated in the control of their function. Immunity (2000) 13:233–42. 10.1016/S1074-7613(00)00023-6 [DOI] [PubMed] [Google Scholar]
- 80. Biber K, Neumann H, Inoue K, Boddeke HWGM. Neuronal “On” and “Off” signals control microglia. Trends Neurosci (2007) 30:596–602. 10.1016/j.tins.2007.08.007 [DOI] [PubMed] [Google Scholar]
- 81. Mott RT, Ait-Ghezala G, Town T, Mori T, Vendrame M, Zeng J, et al. Neuronal expression of CD22: Novel mechanism for inhibiting microglial proinflammatory cytokine production. Glia (2004) 46:369–79. 10.1002/glia.20009 [DOI] [PubMed] [Google Scholar]
- 82. Maciejewski-Lenoir D, Chen S, Feng L, Maki R, Bacon KB. Characterization of fractalkine in rat brain cells: migratory and activation signals for CX3CR-1-expressing microglia. J Immunol (1999) 163:1628–35. [PubMed] [Google Scholar]
- 83. Szepesi Z, Manouchehrian O, Bachiller S, Deierborg T. Bidirectional Microglia–Neuron Communication in Health and Disease. Front Cell Neurosci (2018) 12:323. 10.3389/fncel.2018.00323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Neumann H, Wekerle H. Neuronal Control of the Immune Response in the Central Nervous System: Linking Brain Immunity to Neurodegeneration. J Neuropathol Exp Neurol (1998) 57:1–9. 10.1097/00005072-199801000-00001 [DOI] [PubMed] [Google Scholar]
- 85. Nimmerjahn A, Kirchhoff F, Helmchen F. Neuroscience: Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo . Science (80) (2005) 308:1314–8. 10.1126/science.1110647 [DOI] [PubMed] [Google Scholar]
- 86. Liddelow SA, Marsh SE, Stevens B. Microglia and astrocyte interactions in health and disease: Dynamic Duo or Partners in Crime? Trends Immunol (2020) 41:1–16. 10.1016/j.it.2020.07.006 [DOI] [PubMed] [Google Scholar]
- 87. Liddelow SA, Barres BA. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity (2017) 46:957–67. 10.1016/J.IMMUNI.2017.06.006 [DOI] [PubMed] [Google Scholar]
- 88. Goshi N, Morgan RK, Lein PJ, Seker E. A primary neural cell culture model to study neuron, astrocyte, and microglia interactions in neuroinflammation. J Neuroinflammation (2020) 17:155. 10.1186/s12974-020-01819-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nimmervoll B, White R, Yang JW, An S, Henn C, Sun JJ, et al. LPS-induced microglial secretion of TNFα increases activity-dependent neuronal apoptosis in the neonatal cerebral cortex. Cereb Cortex (2013) 23:1742–55. 10.1093/cercor/bhs156 [DOI] [PubMed] [Google Scholar]
- 90. Wang X, Chen S, Ma G, Ye M, Lu G. Involvement of proinflammatory factors, apoptosis, caspase-3 activation and Ca2+ disturbance in microglia activation-mediated dopaminergic cell degeneration. Mech Ageing Dev (2005) 126:1241–54. 10.1016/j.mad.2005.06.012 [DOI] [PubMed] [Google Scholar]
- 91. Ormel PR, Vieira de Sá R, van Bodegraven EJ, Karst H, Harschnitz O, Sneeboer MAM, et al. Microglia innately develop within cerebral organoids. Nat Commun (2018) 9:1–14. 10.1038/s41467-018-06684-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lin YT, Seo J, Gao F, Feldman HM, Wen HL, Penney J, et al. APOE4 Causes Widespread Molecular and Cellular Alterations Associated with Alzheimer’s Disease Phenotypes in Human iPSC-Derived Brain Cell Types. Neuron (2018) 98:1141–54.e7. 10.1016/j.neuron.2018.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mathews S, Branch Woods A, Katano I, Makarov E, Thomas MB, Gendelman HE, et al. Human Interleukin-34 facilitates microglia-like cell differentiation and persistent HIV-1 infection in humanized mice. Mol Neurodegener (2019) 14:12. 10.1186/s13024-019-0311-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Marschallinger J, Iram T, Zardeneta M, Lee SE, Lehallier B, Haney MS, et al. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat Neurosci (2020) 23:194–208. 10.1038/s41593-019-0566-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Olah M, Patrick E, Villani AC, Xu J, White CC, Ryan KJ, et al. A transcriptomic atlas of aged human microglia. Nat Commun (2018) 9:1–8. 10.1038/s41467-018-02926-5 [DOI] [PMC free article] [PubMed] [Google Scholar]