Abstract
Background
Although many trials are currently investigating the safety and efficacy of convalescent plasma (CP) in critically ill COVID‐19 patients, there is a paucity of ongoing and published studies evaluating the CP donors' side. This retrospective study reports the first Italian experience on CP donors' selection and donations.
Methods
Patients aged 18‐68 years who had recovered from COVID‐19 at least 2 weeks previously were recruited between March 18 and June 30, 2020 in a study protocol at the Italian hospitals of Pavia and Mantova.
Results
During the study period, 494 of 512 donors recruited were judged eligible and underwent 504 plasmapheresis procedures. Eighty‐five percent (437/512) of the CP donors were males. The average time between symptom recovery and CP donation was 36.6 (±20.0) days. Four hundred and eighty‐eight plasmapheresis procedures (96.8%) were concluded and each unit was divided into two subunits (total 976) with an average volume of 316.2 (±22.7) mL. Ninety‐three percent (460/494) of CP donors at the time of plasma donation had a neutralizing IgG titer ≥1:80. Plasmapheresis‐related adverse reactions occurred in 2.6% (13/504) of cases; all the reactions were mild and none required therapeutic intervention. Donors' age and COVID‐19 severity were positively associated with greater antibody responses.
Conclusion
This study demonstrates the feasibility and safety of a pilot CP program conducted in Italy. The identification of factors (ie, age and severity of COVID‐19) positively associated with higher neutralizing antibody titers at the time of donation may help to optimize the selection of CP donors.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19), caused by the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) pandemic, resulted in more than 20 million cases and 800 000 deaths worldwide (data updated on 11 August, 2020). 1 Among the possible anti‐COVID‐19 treatments, the use of plasma from individuals who have recovered from SARS‐CoV‐2 infection has gained increasing interest thanks to preliminary reports on its safety and potential efficacy, particularly when administered early, in critically ill COVID‐19 patients. 2 , 3 , 4 , 5 , 6 The postulated mechanism of action of COVID‐19 convalescent plasma (CP) includes a process of passive immunization through the transfer of specific neutralizing antibodies from recovered individuals to COVID‐19 patients. 7 The interest in CP emerged early during the COVID‐19 pandemic, given the prior use of this strategy to treat other coronavirus diseases (ie, severe acute respiratory syndrome [SARS] and Middle‐East Respiratory Syndrome [MERS]) 8 and the pivotal experiences from China and Italy. 2 , 3 , 4 Following the initial positive clinical experiences of the use of CP in COVID‐19 patients, many European countries and the USA started to organize the collection of hyperimmune plasma to meet current needs and/or to store sufficient numbers of plasma units for future epidemic peaks. 9 , 10 In parallel, several studies evaluating the safety and efficacy of CP have started around the world. 11 Italy was the first country in the western world hit by SARS‐CoV‐2 pandemic with a number of cases and deaths (particularly in the Lombardy region) among the highest in the world (250 000 cases and 35 000 deaths, as of 11 August, 2020) and this generated an unprecedented health and social emergency. 12 Immediately after the first autochthonous SARS‐CoV‐2 cases in Italy, the National Blood Center (CNS) released two documents: the first on measures to prevent the transmission of SARS‐CoV‐2 by transfusion of labile blood components 13 and the second on the criteria for selecting CP donors. 14 Following the latter document, the Italian Transfusion Centers of the University Hospital of Pavia and the City Hospital of Mantova (both located in the Region of Lombardy), developed a multicenter protocol for collection of hyperimmune plasma from donors who had recovered from their coronavirus infection and for use of this plasma to treat critically ill COVID‐19 patients (registered at clinicaltrials.gov as NCT 04321421). 15 In this study we report the results of approximately 3 months' activity (March 18‐30 June 2020) of CP collection at two Italian Transfusion Centers.
2. MATERIAL AND METHODS
2.1. Donor selection and convalescent plasma collection
From 18 March 2020, the Italian Transfusion Centers of the University Hospital of Pavia and later on the City Hospital of Mantova, started to collect hyperimmune plasma from donors who had recovered from COVID‐19 according to the registered and published clinical protocol (NCT 04321421). 15 Donors were recruited according to a hospital‐based call list. Given the emergency period with all medical and nursing staff committed to the care of hospitalized COVID‐19 patients, medical students, recruited in Pavia hospital on a voluntary basis, were involved in the donors' recruitment. After online training according to the instructions given by the Transfusion specialist, the volunteers, working in two daily shifts of 6 hours each, performed a preliminary telephone triage aimed at excluding those individuals not eligible for donation. Individuals who seemed eligible and were potentially interested in being donors were asked to undergo a preliminary blood test to detect anti‐SARS‐CoV‐2 neutralizing antibodies prior to donation (72 hours) to avoid unnecessary donations. Donors were males or females with no previous pregnancies, aged 18 or above, who had completely recovered from COVID‐19 (defined as two consecutive negative polymerase chain reaction tests on naso‐pharyngeal swabs performed 24 hours apart) at least 14 days previously. The protocol was developed according to the current national regulation 14 and donors were clinically evaluated by local physician, with the purpose of highlighting any absolute contraindications to the aphaeresis procedure. All donors needed to test negative for hepatitis A and E RNA, and parvovirus B19 DNA, as well as for hepatitis B and C viruses, human immunodeficiency virus and syphilis at molecular tests. All convalescent donors were tested for anti‐SARS‐CoV‐2 neutralizing antibodies at the time of donation or within 72 hours prior to donation. According to national transfusion laws, all donors were voluntary and unpaid.
Plasma collection was performed in a dedicated facility, using latest‐generation cell separators (Trima Accel ‐Terumo BCT and Amicus ‐ Fresenius Kabi), according to the donors' characteristics, under the supervision of nurses. To prevent hypocalcemia, calcium gluconate was administered intravenously during the procedure, at a mean dose of 1000 mg.
About 660 mL of plasma was collected during each procedure and immediately divided in two bags of equal volume, using a sterile tubing welder. Plasma pathogen reduction was then performed with the INTERCEPT processing system (Cerus Europe BV) or the Mirasol PRT System (Terumo BCT, Lakewood, CO, USA), as specifically required by the CNS, and the products were labeled as hyperimmune COVID‐19 plasma. 14 Finally, the plasma was stored in a dedicated freezer, at a controlled temperature ranging from −80°C to −25°C. Collected CP with a neutralizing titer of 1:80 or higher was validated for clinical use. The neutralization test for the identification of anti‐SARS‐CoV‐2‐neutralizing antibodies was performed at the Molecular Virology Unit of the University Hospital of Pavia and was based on the determination of the cytopathic effect, as previously described. 15 Briefly, 50 μL of diluted serum (4‐fold serial dilutions from 1:10 to 1:640) were added to an equal volume of viral suspension (tissue culture infectious dose of 50 from a SARS‐CoV‐2 strain isolated from a symptomatic patient), incubated for 1 hour at 33°C in 5% CO, and then combined with 3 × 105 Vero‐E6 cells. After incubation for 72 hours, the cells were stained with Gramʼs crystal violet solution. Wells were scored to evaluate the degree of cytopathic effect compared to that of viral controls. The neutralizing titer was the maximum dilution producing a 90% reduction of the cytopathic effect. A titer of ≥1:10 was considered positive.
As per routine, the plasma was biologically validated and made available for infusion at the completion of all tests. Requests for ABO compatible hyperimmune plasma were made by treating physician using the established local procedures, including electronic tracking. The following characteristics of the convalescent donors and their plasma were electronically collected: age, sex (male/female), weight and body mass index (BMI), eligibility for plasma donation, causes of ineligibility, time interval between recovery from symptoms and plasma donation, clinical severity of COVID‐19 (1 = asymptomatic subjects, 2 = subjects managed as outpatients, 3 = subjects requiring hospital admission, 4 = subjects requiring hospital admission and mechanical respiratory support), number of plasma units collected and time interval between donations, number of plasmaphereses interrupted and causes, volume of the plasma unit collected, donors' neutralizing titer detected at plasma donation, number of plasma units eliminated and causes, plasma sub‐units produced from each plasma donation, and temperature of storage. Finally, adverse reactions in the donors were recorded and classified according to their severity: no symptoms, mild symptoms not requiring therapeutic intervention, symptoms requiring therapeutic intervention and symptoms requiring resuscitation procedures. The outcome of any adverse reactions (data unavailable, complete resolution within a few hours, complete resolution within a few days, persistence of sequelae) was also recorded. The protocol for donor selection and plasma collection and testing was approved by the Local Ethical Committees of the hospitals in Pavia and Mantova. All CP donors gave signed informed consent prior to donation.
2.2. Statistical analysis
All continuous variables are summarized using the mean and standard deviation (SD) or the median and the interquartile range (IQR). The frequency and percentage are reported for all categorical measures. An exploratory analysis was performed with a Spearmanʼs correlation matrix including all variables, using complete observations only. For this analysis “severity” was considered a quantitative variable. The correlation matrix was depicted by weighted network visualization. Partial correlations were also calculated and depicted in a similar way. Since partial correlations were based on direct adjusted effects, they were candidates for attempting to create a causal network. A causal plot was generated using the R package “bnlearn” by Scutari. 16 Starting from raw data, a Bayesian network was produced. Bayesian networks are graphical models in which nodes represent random variables and arrows represent probabilistic dependencies. However, some constraints were applied, according to the results of the partial correlations and common‐sense knowledge. 17 The causal network prompted a marginal correlation analysis involving age, clinical severity and neutralizing antibody titer, in which the indirect and direct effects of age on antibody titer were evaluated as average marginal effects. The dataset consisted of 512 observations, each concerning a different patient who had recovered from COVID‐19; all patients donated hyperimmune plasma. The following variables were considered: “age,” sex (binary variable: female = 1, male = 0), “interval” (number of days elapsed from clinical recovery and convalescent plasma collection), clinical severity (“severity”, coded as 1 to 4), body mass index (“BMI”), and neutralizing antibody titer. The variable “severity” was also recoded in a binary form as “hi_sev” = 0 (corresponding to “severity” ≤2) or “hi_sev” = 1 (corresponding to “severity” >2). The variable “age” was also recoded as a discrete variable (“agecat”) for some estimations, cutting at 10‐year intervals, from 20 to 70 years. The neutralizing antibody titer was recoded as log2(dilutions/10), as “l2tit,” when a titer <1/10 (l2tit −1) was assumed to be 5 dilutions, a titer 1/10 (l2tit 0) corresponded to 10 dilutions, a titer 1/20 (l2tit 1) to 20 dilutions, a titer 1/40 (l2tit 2) to 40 dilutions, a titer 1/80 (l2tit 3) to 80 dilutions, up to a titer 1/640 (l2tit 6) which corresponded to 640 dilutions. These conversion steps from the original antibody to l2tit were utilized for the statistical analysis. The predictive effect of “age” on “l2tit” was evaluated by analysis of variance (ANOVA), using “agecat.” The predictive effect of “severity” on “l2tit” was evaluated by ANOVA, as was the relation between “age” and “severity.” Pairwise comparisons of the marginal linear predictions were calculated. The predictive effect of “age” on clinical “severity” was evaluated by an ordered logistic regression method. The predictive effect of “severity” on “interval” was evaluated by ANOVA. The pairwise comparisons of the marginal linear predictions were calculated. Estimates were obtained using R version 4.0.2 and Stata 16.1.
3. RESULTS
From 18 March 2020 to 30 June 2020, a total of 512 patients who had recovered from COVID‐19 were evaluated as potential COVID‐19 CP donors. The demographics, baseline and clinical characteristics of these potential donors are reported in Table 1. All the donors were of Caucasian origin. Eighteen (3.5%) were not eligible for plasma donation according to the selection criteria established by the protocol: 12 because of their personal history (seven had traveled in malaria‐endemic areas and five had risk behaviors for sexually transmitted diseases) and six for clinical reasibs (three cardiovascular disorders, two autoimmune disorders and one cancer). The population of potential CP donors had a mean age of 47.7 years and consisted of more males (85%) than females, because of the restriction of CP donation only to women who had not had previous pregnancies to prevent transfusion‐related acute lung injury (TRALI). An increased mean weight (83.2 ± 14.5 kg) and BMI (26.9 ± 5.5) were also recorded among the CP donors. Approximately two‐thirds of the evaluable patients (239/358, 66.8%) were classified as having had mild‐to‐moderate disease not requiring hospitalization, while a minority of them had been asymptomatic (13/358, 3.6%). Eighty‐six percent (427/494) of CP donors were first‐time plasmapheresis donors. The average time interval between recovery from symptoms and CP donation was 36.6 (±20) days (Table 1). Among the 494 analyzed CP donors, a neutralizing titer of 1:80 was detected in the majority of cases (282/494, 57.1%), while 36% of them (178/494) had a titer ≥1:160 and only a minority of them had a titer ≥1:640 (18/494, 3.6%). A total of 504 plasmapheresis procedures were performed in the 494 eligible CP donors. Ninety‐eight percent (484/494) of CP donors underwent one plasmapheresis procedure, while the remaining 10 patients underwent two plasmapheresis procedures. All but 16 procedures (488/504, 96.8%) were completed. The causes of interruption were adverse reactions to the plasmapheresis procedure or donor compliance issues (anxiety and fear). Thirteen donor adverse reactions (13/504, 2.6%) were reported during or immediately following the plasmapheresis procedures. They were all mild (seven hypotension, three immediate vasovagal reactions and three venous access rupture) and resolved completely within a few hours from onset without requiring therapeutic intervention.
TABLE 1.
Demographics, baseline and clinical characteristics of 512 convalescent plasma donors
| Age (y) | |
| Mean ± SD | 47.7 ± 11.8 |
| IQR | 39‐56 |
| ≤29 | 40 (7.8%) |
| 30‐39 | 96 (18.8%) |
| 40‐49 | 138 (27.1%) |
| ≥50 | 238 (46.3%) |
| Sex | |
| Male | 437 (85.3%) |
| Female | 75 (14.7%) |
| Weight (kg) | |
| Mean ± SD | 83.2 ± 14.5 |
| IQR | 71‐90 |
| Body mass index | |
| Mean ± SD | 26.9 ± 5.5 |
| IQR | 23‐29 |
| Degree of severity of disease | |
|
13 (2.5%) 239 (46.7%) 93 (18.3%) 31 (6%) |
| Data not available | 136 (26.5%) |
| Time interval between symptom resolution and donation (d) | |
| Mean ± SD | 36.6 ± 20.0 |
| IQR | 12‐49 |
| Not eligible donors | N = 18 |
| Anamnestic criteria | 12 (66.6%) |
| Clinical criteria | 6 (44.4%) |
| Neutralizing titer detected among CP donors | N = 494 |
| <1:80 | 22 (4.4%) |
| 1:80 | 282 (57.1%) |
| 1:160 | 113 (22.9%) |
| 1:320 | 47 (9.5%) |
| ≥ 1:640 | 18 (3.6%) |
| Data not available | 12 (2.5%) |
| Total number of plasmapheresis procedures | N = 504 |
| Number of completed procedures | 488 (96.8%) |
| Number of interrupted procedures | 16 (3.2%) |
| Cause of interruption: | |
|
13 (81.2%) |
|
3 (18.8%) |
| Donor adverse reactions | N = 13 |
| Hypotension | 7 (54%) |
| Immediate vasovagal reaction | 3 (23%) |
| Venous access rupture | 3 (23%) |
Abbreviations: CP, convalescent plasma; IQR, interquartile range; SD, standard deviation.
The characteristics of the 488 CP units collected are reported in Table 2. The average volume of CP collected by plasmapheresis was 641.5 (±96.8) mL, while the average volume of the 976 plasma subunits produced from the CP plasmapheresis units was 316.24 (±22.7) mL. All CP units were frozen within 6 hours of collection and stored at a temperature ≤−40°C. Overall, 37 CP units (7.6%) were eliminated. The main cause of CP elimination was a low (<1:80) neutralizing titer (22/37, 59.5%), followed by positivity at viral screening (9/37, 24.3%; six parvovirus B19 and three hepatitis C virus).
TABLE 2.
Characteristics of the convalescent plasma units collected
| Number of convalescent plasma units collected | 488 |
|---|---|
| Volume of the plasma units collected (mL) | |
| Mean ± SD | 641.5 ± 96.8 |
| IQR | 600‐660 |
| Number of eliminated plasma units | 37 |
| Cause of elimination: | |
|
6 (16.2%) |
|
3 (8.1%) |
|
3 (8.1%) |
|
1 (2.7%) |
|
22 (59.5%) |
|
1 (2.7%) |
|
1 (2.7%) |
| Number of plasma subunits produced from each plasma donation | 2 |
| Total number of subunits produced | 976 |
| Volume of the plasma subunits produced (mL) | |
| Mean ± SD | 316.24 ± 22.7 |
| IQR | 300‐340 |
Abbreviations: HCV, hepatitis C virus; HLA, human leukocyte antigen; IQR, interquartile range; SD, standard deviation.
The correlation and partial correlation matrices pertaining to the relationship between the different variables considered (ie, age, sex, disease severity, BMI, interval between clinical recovery and CP collection, neutralizing antibody titer) are shown in Figure 1A,B, respectively. In the partial correlation matrix the direct link between “age” and “l2tit” was weakened, but not suppressed. This suggests that the effect of “age” on “l2tit” was partly direct and partly mediated by “severity”. Moreover, the direct arcs between “female” and “interval”, and between “female” and “l2tit” lost significance. On the basis of these results, a causal interpretation was proposed (Figure 2). Figure 2 illustrates the resulting causal model, focusing only on the net effect of “age” on antibody response “l2tit”. As stated above, this effect was partially mediated by disease “severity,” and was partially direct. The group of “age”, “severity”, and “l2tit” comprised the components of the “causal effect” of interest to us. The proportion of the indirect effect of “age” on “l2tit”, mediated by “hi_sev,” was 28.33% of the total effect (total effect = indirect + direct effect, age on l2tit without any mediation).
FIGURE 1.
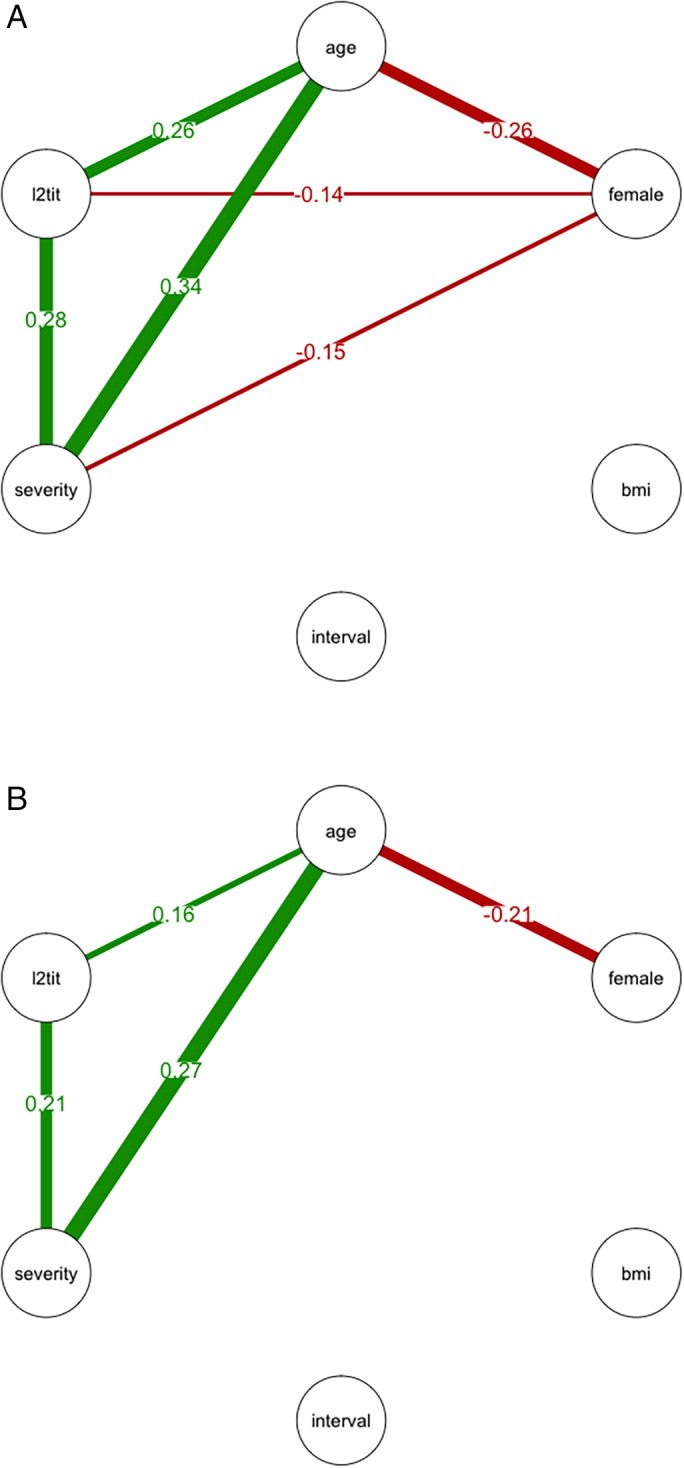
Correlation (A) and partial correlation matrices (B) illustrated by weighted network visualization. The nodes are the variables, and the connectors (“arcs” or “edges”) are the pairwise correlations. Green color indicates positive correlations, red color indicates negative correlations, and numbers express the value of the correlations. Non‐significant arcs were omitted [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 2.
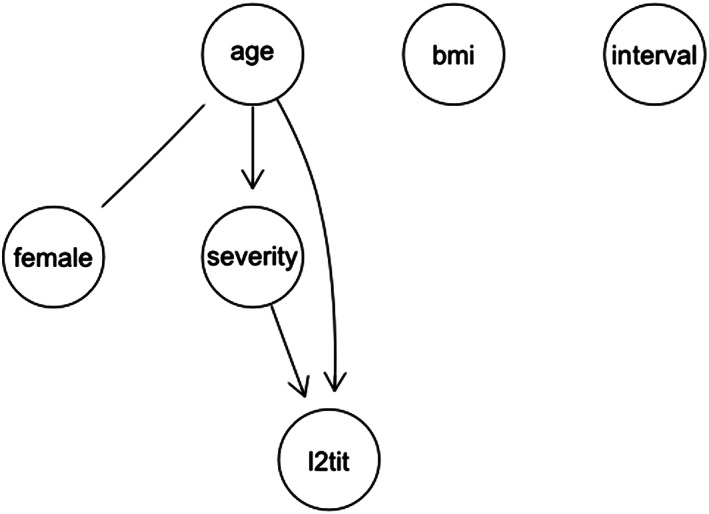
Proposed causal interpretation of correlation between variables. The directed arcs (arrows) indicate causal effects. The undirected edge marks an unresolved correlation (possibly joint dependence on an unknown variable)
The predictive effect of the variables are reported in Figures 3, 4, 5 and Figure S1. The predictive effect of age on neutralizing antibody titer is depicted in Figure 3. The antibody titer increased significantly with age (P = .0001) (number of observations = 355). In addition, the antibody titer increased with clinical severity. ANOVA on 359 observations demonstrated a significant effect of disease severity on neutralizing antibody titer (P = .0000). The marginal effects are depicted in Figure 4. Levels 3 and 4 of severity were associated with significantly higher antibody titers than levels 1 and 2; the difference between levels 3 and 4 was not statistically significant. The predictive influence of age on clinical severity was significant (P = .0000) and is shown in Figure 5. The probability of severity = 1 or 2 appeared to decrease at higher ages, the opposite happened with severity = 3 or 4 (number of observations = 376). Finally, at ANOVA the clinical severity was significantly predictive (P = .0020) of the time interval between clinical recovery and CP donation (Figure S1). The effect was largely due to severity = 4, that is, to patients needing respiratory assistance (number of observations = 375).
FIGURE 3.
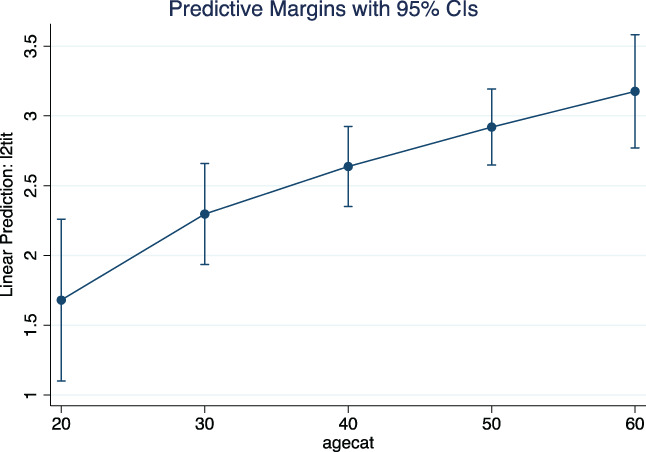
Predictive effect of age (agecat) on neutralizing antibody titer (l2tit) [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 4.
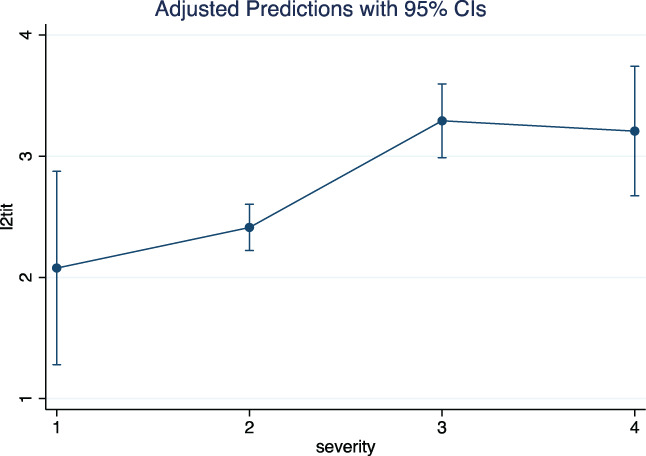
Predictive effect of clinical severity of the disease on neutralizing antibody titer (l2tit) [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 5.
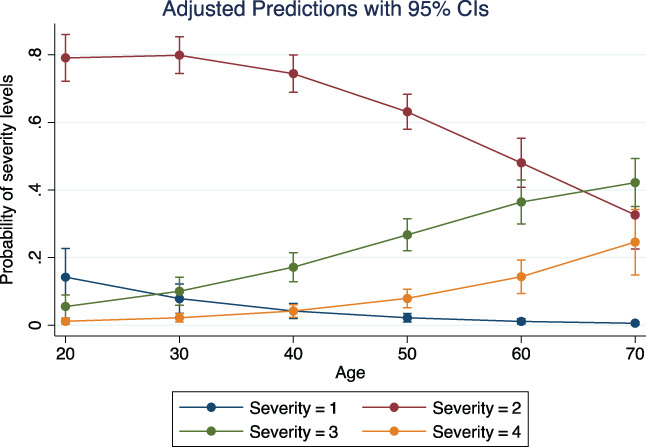
Logistic regression on the predictive effect of age on probability of clinical severity. The lower levels, 1 and 2, decline with advancing age, whereas the opposite appears with the higher levels [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
Italy was the first western country to suffer the severe impact of the COVID‐19 pandemic as it spread from China. 12 The Italian Region of Lombardy, which has a population (about 11 million people) similar to that of Wuhan, China, in which the SARS‐CoV‐2 epidemic was initially focused, was devastated by an infectious wave similar to a “tsunami” at the end of February, 2020. 13 Thousands of severely symptomatic COVID‐19 patients requiring hospital admission arrived contemporaneously at the emergency rooms of many Lombard hospitals, quickly overcoming their hospitalization capacities. In this dramatic situation, considering the scarce therapeutic resources available at that time, we decided to develop a protocol for the collection and infusion of CP to critically ill COVID‐19 patients. 15 This idea loomed following the pioneering encouraging experience from China and the positive results on the use of CP during previous viral epidemics, including SARS and MERS coronavirus diseases. 18
As soon as the first CP donors were available, we started a pilot program of CP collection according to the timely and rigorous criteria provided by the CNS for CP donor selection. 14 Given the shortage of specialized hospital personnel who, like others, 9 were all engaged in the management of the emergency, in Pavia hospital the realization of this program was possible thanks to the involvement of medical students who were trained in CP donor recruitment and selection. In fact, although the protocol set out that donors should have been recruited on the basis of a call list established by the specialists of the Infectious Diseases Unit of the participating institutions, the impact of the workload on the staff made this impossible. Medical students represented the best and immediate solution at that time. The dramatic situation led us to decide that setting up a call center was the best choice, since Pavia hospital was receiving hundreds of telephone calls from people requesting information about plasma donation. In our opinion this “direct communication” between medical students and patients who had recovered from their infection had a positive impact on both: immediate human contact for physically and psychologically stressed people and a “training ground for life” for the future doctors.
The Italian experience of the two transfusion centers of Pavia and Mantova, presented in this report, is the largest study published so far and demonstrates the feasibility and safety of our CP donor selection strategy. 19 , 20 , 21 Indeed, among the 512 CP potential donors initially enrolled, only 3.5% were not eligible for CP donation (18/512, 12 for criteria related to their personal history and six because of clinical criteria). Similarly, only 3.2% of plasmapheresis procedures were not completed (16/504, 13 because of donor adverse reactions and three because of donor compliance issues). Adverse reactions were rare, occurring in only 2.6% (13/504) of cases, and all of them were mild and resolved spontaneously, documenting the feasibility of this CP apheresis collection program. These safety data, available for the first time in CP donors, reveal slightly increased rates of adverse reactions than those reported in Italian apheresis donors in 2019 (4.9 per 1000 apheresis donations procedures). 22 This increased incidence is, however, not surprising considering that most CP donors (86%) are occasional donors, who have just recovered from COVID‐19, and not committed repeat blood donors. Approximately half of the CP subunits donated were transfused to COVID‐19 patients and, based on national hemovigilance data, no safety concerns have emerged in recipients during and following CP transfusion (unpublished data).
Regarding the statistical analysis, two different complementary approaches were taken. The first (network analysis) was innovative and aimed mainly at highlighting causal connections between different variables; the second was classical (logistic regression analysis and ANOVA) and used to evidence the predictive values of variables on each other. Whichever statistical method was used, age, clinical severity, and antibody titer were found to be tightly associated in a positive way. A significant effect of age on COVID‐19 severity had already been established in other studies. 23 , 24 For instance, in an age‐structured mathematical model of epidemic data from China, Italy, Japan, Singapore, Canada and South Korea, Davies and colleagues 23 observed an age‐specific clinical susceptibility to COVID‐19 (69% of infected individuals aged over 70 years manifested clinical symptoms as compared with 21% of infected individuals aged 10 to 19 years). Female sex seemed to act negatively on antibody titer with the mediation of age. Indeed, there were fewer female patients than male ones, so there was a negative correlation between female sex and age. On the other hand, sex did not appear to have a direct influence on clinical severity or antibody titer. The positive correlation between the interval from symptom resolution to plasma donation and disease severity, demonstrated by the ANOVA method, was mainly due to the delay in CP donation from patients needing respiratory assistance. These findings are in keeping with those from Klein and colleagues, 21 who observed, in a population of 126 CP donors, that age and hospitalization were associated with higher convalescent antibody titers. A similar association between COVID‐19 severity and neutralizing antibody titers was also observed in blood donors from the initial area of lockdown in the province of Lodi. 25
In conclusion, this study demonstrates for the first time the feasibility and safety for donors of a pilot program of CP donation conducted in Italy. In our opinion the results are particularly relevant considering the large number and homogeneity of the Italian CP donor population enrolled and the paucity of published data evaluating the characteristics of CP donors and donations, including the safety issue. In addition identifying those factors (ie, severity of COVID‐19 disease and age) positively associated with the likelihood of having higher levels of neutralizing IgG antibodies at the time of CP collection, may help investigators to define more selective and effective criteria for CP donor recruitment and donation. Thus, considering the current broad spread of the SARS‐CoV‐2 around the world and the still lack of preventive measures or treatment for COVID‐19, including vaccines and hyper‐immune immunoglobulin, national governments and health authorities should implement CP collection programs in parallel with randomized controlled trials aimed at determining the safety and efficacy of hyperimmune plasma administered to COVID‐19 patients.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
AUTHOR CONTRIBUTIONS
Claudia Del Fante, Fausto Baldanti, Elena Percivalle, Claudia Glingani, Cristina Mortellaro, and Gianluca Viarengo were responsible for donor recruitment, plasma collection and performing assays; Giuseppe Marano, Carlo Mengoli and Massimo Franchini were responsible for data analysis and writing the manuscript; Cesare Perotti and Giancarlo Maria Liumbruno contributed to the study design and revising the manuscript.
Supporting information
Figure S1. Predictive effect of disease clinical severity on interval (days) between clinical recovery and convalescent plasma donation.
ACKNOWLEDGMENTS
The authors especially thank all the individual who, with civic sense, agreed to be immediately available to donate plasma.
The Pavia Hospital is very grateful to Prof Giambattista Parigi (medical student coordinator) and all the medical students for their valuable help in recruiting the donors, who had recovered from COVID‐19, during the emergency in Lombardy region.
This research was supported by funds from the Fondazione IRCCS Policlinico San Matteo, Ricerca Corrente grant no. 80206 and from the European Commission ‐ Horizon 2020 (EU project 101003650 ‐ ATAC).
Del Fante C, Franchini M, Baldanti F, et al. A retrospective study assessing the characteristics of COVID‐19 convalescent plasma donors and donations. Transfusion. 2021;61:830–838. 10.1111/trf.16208
Funding information European Commission ‐ Horizon 2020, Grant/Award Number: 101003650; Fondazione IRCCS Policlinico San Matteo, Grant/Award Number: 80206
REFERENCES
- 1. WHO . Coronavirus disease (COVID‐2019). Situation reports August 2020. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200807-covid-19-sitrep-200.pdf?sfvrsn=2799bc0f_2. Accessed August 7, 2020.
- 2. Perotti C, Baldanti F, Bruno R, et al. Mortality reduction in 46 patients with severe COVID‐19 treated with hyperimmune plasma. A proof‐of‐concept, single‐arm, multicenter trial. Haematologica. 2020. 10.3324/haematol.2020.261784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID‐19 with convalescent plasma. JAMA. 2020;323:1582–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID‐19 patients. Proc Natl Acad Sci USA. 2020;117:9490–9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zeng QL, Yu ZJ, Gou JJ, et al. Effect of convalescent plasma therapy on viral shedding and survival in COVID‐19 patients. J Infect Dis. 2020;222:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Joyner MJ, Wright RS, Fairweather D, et al. Early safety indicators of COVID‐19 convalescent plasma in 5,000 patients. J Clin Invest. 2020;130(9):4791–4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rojas M, Rodríguez Y, Monsalve DM, et al. Convalescent plasma in Covid‐19: Possible mechanisms of action. Autoimmun Rev. 2020;19:102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xie M, Chen Q. Insight into 2019 novel coronavirus ‐ an updated interim review and lessons from SARS‐CoV and MERS‐CoV. Int J Infect Dis. 2020;94:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Budhai A, Wu AA, Hall L, et al. How did we rapidly implement a convalescent plasma program? Transfusion. 2020;60:1348–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. European Blood Alliance . [cited 2020 Oct 8]. Available from: https://europeanbloodalliance.eu/covid-19-a-european-project-will-study-the-use-of-convalescent-plasma-as-a-treatment/.
- 11. Murphy M, Estcourt L, Grant‐Casey J, Dzik S. International survey of trials of Convalescent plasma to treat COVID‐19 infection. Transfus Med Rev. 2020;34(3):151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Health Organization . Coronavirus disease (COVID‐19) outbreak. [cited 2020 Nov 8]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- 13. Franchini M, Farrugia A, Velati C, et al. The impact of the SARS‐CoV‐2 outbreak on the safety and availability of blood transfusions in Italy. Vox Sang. 2020;115:603–605. 10.1111/vox.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Franchini M, Marano G, Velati C, Pati I, Pupella S, Maria Liumbruno G. Operational protocol for donation of anti‐COVID‐19 convalescent plasma in Italy. Vox Sang. 2020. 10.1111/vox.12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perotti C, Del Fante C, Baldanti F, et al. Plasma from donors recovered from the new coronavirus 2019 as therapy for critical patients with COVID‐19 (COVID‐19 plasma study): A multicentre study protocol. Intern Emerg Med. 2020;15(5):819–824. 10.1007/s11739-020-02384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scutari M. Learning Bayesian networks with the bnlearn R package. J Stat Softw. 2010;35:1–22.21603108 [Google Scholar]
- 17. Vlek CS, Prakken H, Renooij S, Verheij B. A method for explaining Bayesian networks for legal evidence with scenarios. Artif Intell Law. 2016;24:285–324. [Google Scholar]
- 18. Wooding DJ, Bach H. Treatment of COVID‐19 with convalescent plasma: lessons from past coronavirus outbreaks. Clin Microbiol Infect. 2020;26(10):1436–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li L, Yang R, Wang J, et al. Feasibility of a pilot program for COVID‐19 convalescent plasma collection in Wuhan, China. Transfusion. 2020;60:1773–1777. 10.1111/trf.15921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li L, Tong X, Chen H, et al. Characteristics and serological patterns of COVID‐19 convalescent plasma donors: optimal donors and timing of donation. Transfusion. 2020;60(8):1765–1772. 10.1111/trf.15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klein SL, Pekosz A, Park HS, et al. Sex, age, and hospitalization drive antibody responses in a COVID‐19 convalescent plasma donor population. J Clin Invest. 2020;130(11):6141–6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Catalano L, Piccinini V, Pati I, et al. CNS Report/2020. Italian Blood System 2019: activity data, haemovigilance and epidemiological surveillance. Volume 1. 2020. Available from: https://www.centronazionalesangue.it/sites/default/files/Italian%20Blood%20System%202019%20Vol.%201.pdf#overlay‐context=node/90. Accessed September 2, 2020.
- 23. Davies NG, Klepac P, Liu Y, et al. Age‐dependent effects in the transmission and control of COVID‐19 epidemics. Nat Med. 2020;26:1205–1211. [DOI] [PubMed] [Google Scholar]
- 24. Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID‐19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Percivalle E, Cambiè G, Cassaniti I, et al. Prevalence of SARS‐CoV‐2 specific neutralising antibodies in blood donors from the Lodi Red Zone in Lombardy, Italy, as at 06 April 2020. Euro Surveill. 2020;25:2001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Predictive effect of disease clinical severity on interval (days) between clinical recovery and convalescent plasma donation.


