Abstract
Perfluorooctanoic acid (PFOA) is a per- and polyfluoroalkyl substance (PFAS) once used as a surfactant in the polymerization of chemicals. Because of its ubiquitous nature and long half-life, PFOA is commonly detected in the environment, wildlife, and humans. While skin exposure to PFOA is of concern, studies evaluating the immunotoxicity of dermal exposure are lacking. These studies evaluated the immunotoxicity of PFOA (0.5–2% w/v, or 12.5–50 mg/kg/dose) following dermal exposure using a murine model. PFOA (0.5–2%) was not identified to be an irritant or sensitizer using the local lymph node assay. The IgM antibody response to sheep red blood cell. was significantly reduced in the spleen following 4-days of dermal exposure (2%). PFOA exposure produced a significant decrease in thymus (1 and 2%) and spleen (0.5–2%) weight along with an increase in liver weight (0.5–2%). Immune cell phenotyping identified a reduction in the frequency (1 and 2%) and number (0.5–2%) of splenic B-cells. To further define the mechanism of immunotoxicity, gene expression was also evaluated in the skin. The findings support a potential involvement of the nuclear receptor PPARα. These results demonstrate that dermal exposure to PFOA is immunotoxic and raise concern about potential adverse effects from dermal exposure.
Keywords: Allergy, Immune suppression, Immunotoxicity, Perfluorooctanoic acid
1. Introduction
Perfluorooctanoic acid (PFOA) is a per- and polyfluoroalkyl substance (PFAS) once widely used beginning in the 1950s primarily as a surfactant in the polymerization of chemicals including fluoroacrylic esters, fluoropolymers, and fluoroelastomers. Commercial applications pertinent to these processes include manufacturing of flame retardants and extinguishers, surfactants, waxes and gloss finish enhancers, and water repellants in fabrics (Kudo et al., 2003; Starkov et al., 2002). Industrial production and emission of PFOA in North America and Europe was phased-out beginning in the early 2000s, with a complete cessation of PFOA production in the U.S. being achieved by 2015 (ITRC, 2017). Correspondingly, serum levels evaluated in human study populations have declined over time in these regions (Herrick et al., 2017). However, production of these chemicals or their precursors has increased in parts of Asia, and materials imported to the U.S. may still contain PFOA and/or related precursors. The hydrolytic half-life of PFOA in the environment is estimated to be greater than 97 years and the biological half-life in humans is reported to be 4.37 years (Kudo et al., 2003). Because of its ubiquitous nature, PFOA is commonly detected in the environment, wildlife, and humans (Giesy et al., 2001; Kannan et al., 2001, 2002a, 2002b, 2002c; Nakata et al., 2006). Additionally, PFOA has been identified in the blood of occupationally exposed individuals (Kudo et al., 2003; Ubel et al., 1980), as well as the general population (Emmett et al., 2006a, 2006b; Olsen et al., 1998, 2003). Occupational surveillance has demonstrated an increase in PFOA levels in the serum of individuals with and without workplace exposure; while several studies observed no discernable health effects (Olsen et al., 2003b; Olsen et al., 1998) the majority of evidence associates PFOA exposure with detrimental health outcomes including associations with numerous cancers (testicular and kidney), organ toxicity (hepatic, renal, etc.), endocrine, reproductive, developmental, and immunological effects (ATSDR, 2018; Gilliland et al., 1996; Kudo et al., 2003). Much of what we know about PFOA-mediated health effects was generated from epidemiological studies on occupationally exposed individuals with increased levels of PFOA present in their blood (Olsen et al., 2007) and individuals exposed to high environmental concentrations of PFOA (Barry et al., 2013; Frisbee et al., 2009).
The long clearance half-life of PFOA in humans has provoked intense interest in understanding the potential associated human health effects (Chang et al., 2016). Largely guided by evidence of immunotoxic effects of PFOA in in vitro and in vivo experimental systems, much epidemiologic research in recent years has focused on the possible effects of these chemicals on the immune system. Numerous reports have demonstrated PFOA-induced organ (liver, thymus, spleen) and systemic (body weight) toxicity following oral exposure (Betts, 2007; Kudo et al., 2003), in various animal models. Other adverse effects commonly reported in experimental animals exposed to PFOA include: carcinogenicity, hepatomegaly and hepatocyte proliferation, hormone disruption, and a myriad of developmental effects, including neonatal mortality (DeWitt et al., 2009b). PFOA is also immunosuppressive following oral exposure in murine models. A reduction in thymocyte and splenocyte populations, altered T lymphocyte populations, and an inhibition of T-cell-dependent IgM and IgG antibody responses have been documented (DeWitt et al., 2009a; Dewitt et al., 2008; DeWitt et al., 2009b; Yang et al., 2002; Yang et al., 2001). In addition to its immunosuppressive effects, it was also demonstrated that dermal exposure to PFOA, although not allergenic by itself, enhances ovalbumin (OVA)-induced IgE production and airway hypersensitivity in a murine model (Fairley et al., 2007b). These data suggest that PFOA has the potential to augment IgE-mediated responses induced by other environmental and occupational allergens.
The potential for dermal exposure to PFOA is high, both during the manufacturing process, as well as in commercial products such as firefighting foams and carpet and fabric protectants. As one of the major uses of PFOA has been in carpet and fabric protectants, the potential exists for children to be exposed through dermal contact (as well as hand to mouth contact) and adults through both environmental and occupational exposures (Begley et al., 2005) (Kubwabo et al., 2005). Also, due it its persistance there is also concern for exposure in ground water which could occur during bathing or swimming, especially near sites where production once occured. (EPA, 2019). Despite the potential for dermal exposure, most research has focused on the immunotoxic effects of oral and inhalation PFOA exposure.
Previous data published in our laboratory suggest that PFOA is dermally absorbed and that under certain conditions the skin may be a significant route of exposure (Franko et al., 2012). Other than our previous findings, and a report of occupational dermal absorption in Chemolite workers (EPA, 2002), most investigations have ignored dermal PFOA exposure due to the assumption that it is not well absorbed by the skin (EPA, 2002). With the exception of a few older studies and unpublished reports that have conducted limited research looking at dermal irritancy and sensitization following PFOA exposure (Kennedy et al., 2004), there is an overall lack of data regarding the toxicity and/or immunogenicity of dermal exposure. Since the potential for PFOA dermal exposure still exists due to its environmental persistence, it is important to fully understand the potential for dermal penetration and the risk from exposure to PFOA through the dermal route.
In these studies, we show that dermal PFOA exposure induces immunotoxicity in a murine model, similar to that reported for oral and dietary exposure, and begin to define the associated mechanisms. These findings raise additional concerns about the immunotoxicity of PFOA and the dermal exposure route. In view of the ongoing environmental release and persistence of PFOA, widespread detection in humans, experimental and epidemiological evidence of immunotoxicity following oral exposure, and lack of data from the dermal route of exposure, these additional data enrich the database with respect to the immune hazard of PFOA.
2. Materials and methods
2.1. Test articles and chemicals
Acetone [CAS #67–64-1], perfluorooctanoic acid (PFOA; 96%) [CAS #335–67-1], α-hexylcinnamaldehyde (HCA) [CAS# 101–86-0], and cyclophosphamide [CP; CAS# 50–18-0] were purchased from Aldrich Chemical Company, Inc. (Milwaukee, WI).
2.2. Species selection
Female BALB/c and B6C3F1 mice were used in these studies. BALB/c mice have a T-helper (TH)-2 bias and are commonly used to evaluate potential IgE-mediated sensitization, and were therefore used in the hypersensitivity studies (Klink et al., 2003; Woolhiser et al., 2000). B6C3F1 mice are the strain of choice for immunotoxicity studies and were used to evaluate the IgM response to sheep red blood cells (SRBC) (Luster et al., 1992).
All mice were purchased from Taconic (Germantown, NY) at 6–8 weeks-of-age. Upon arrival, the animals were allowed to acclimate for a minimum of 5 days. Each shipment of animals was randomly assigned to treatment group, weighed, and individually identified via tail marking using a permanent marker. A preliminary analysis of variance on body weights was performed to insure a homogeneous distribution of animals across treatment groups. The animals were housed at a maximum of 5 mice/cage in ventilated plastic shoebox cages with hardwood chip bedding. NIH-31 modified 6% irradiated rodent diet (Harlan Teklad) and tap water was provided from water bottles, ad libitum. The temperature in the animal facility was maintained between 68 and 72°F and the relative humidity between 36 and 57%; a light/dark cycle was maintained at 12-h intervals. All animal experiments were performed in an AAALAC International accredited National Institute for Occupational Safety and Health (NIOSH) animal facility in accordance with an animal protocol approved by the Institutional Animal Care and Use Committee.
2.3. PFOA exposures
For the sensitization study, BALB/c mice (5 mice/group) were topically treated on the dorsal surface of each ear with acetone vehicle, increasing concentrations of PFOA (0.5–2% w/v, or 12.5–50 mg/kg/dose) or positive control [30% HCA (v/v; sensitization positive control) once a day for three consecutive days. For the immune phenotyping and gene expression studies, BALB/c mice (5 mice/group) were topically exposed to acetone or increasing concentrations of PFOA (0.5–2% w/v, or 12.5–50 mg/kg/dose) on the dorsal surface of each ear once a day for 4 or 14 days. For analysis of the IgM response to SRBC, B6C3F1 mice (N = 5) were topically exposed to acetone or increasing concentrations of PFOA (0.5–2%) on the dorsal surface of each ear once a day for 4 consecutive days. Cyclophosphamide (20 mg/kg in isotonic sterile saline) was included as the positive control for the analysis of the IgM response to SRBC and was injected intraperitoneally 4 days prior to sacrifice.
2.4. Combined local lymph node and irritancy assay
To determine the irritancy and sensitization potential of PFOA, a combined local lymph node assay (LLNA) was conducted according to the methods previously described (Anderson et al., 2013a). PFOA dosing concentrations (0.5–2% w/v, or 12.5–50 mg/kg/dose) and vehicle (acetone) were selected based on solubility and preliminary concentration range finding studies. HCA (30%) was included as the assay positive control.
2.5. Spleen in vivo response to the T-cell-dependent antigen SRBC
The primary IgM response to sheep red blood cells (SRBC) was enumerated using a modified hemolytic plaque assay of Jerne and Nordin (1963). Four days prior to euthanasia, the mice were immunized with 7.5 × 107 SRBC (in 200 μl volume) by intravenous injection. All SRBC for these studies were drawn from a single donor animal (Lampire Laboratories, Pipersville, PA). On the day of sacrifice, mice were euthanized by CO2 asphyxiation, body and organ weights were recorded, and spleens were collected in 3 mL of Hank's balanced salt solution (HBSS). Single cell suspensions of the spleens from individual animals were prepared in HBSS by disrupting the spleen between the frosted ends of microscopic slides. To identify the total number of spleen cells, 20 μl of cells were added to 10 mL of Isoton II diluent (1:500; Beckman Coulter, Brea, CA) and two drops of Zap-o-globin (Beckman Coulter, Brea, CA) were added to lyse red blood cells. Cells were then counted in the Coulter counter. Dilutions (1:30 and 1:120) of spleen cells were then prepared and 100 μl of each dilution were added to test tubes containing a 0.5 mL warm agar/dextran mixture (0.5% Bacto-Agar, DIFCO; and 0.05% DEAE dextran; Sigma, St. Louis, MO), 25 μl of 1:1 ratio of SRBC suspension, and 25 μl of 1:4 dilution (1 mL lyophilized) guinea pig complement (Cedarlane Labs, Burlington, Canada). Each sample was vortexed, poured into a petri dish, covered with a microscope coverslip, and incubated for 3 h at 37 °C. The plaques (representing antibody-forming B-cells) were then counted. Results were expressed in terms of both specific activity (IgM PFC per 106 spleen cells) and total activity (IgM PFC per spleen).
2.6. Immune phenotyping
Animals were euthanized by CO2 inhalation 24 h after the final exposure, weighed, and examined for gross pathology. The liver, spleen, kidneys, and thymus were removed, cleaned of connective tissue and weighed. Left and right auricular draining lymph nodes (DLNs; drain the site of chemical application) and spleen were collected in 4 mL sterile phosphate-buffered saline (pH 7.4). Spleen and DLN cell suspensions (2 nodes/animal) were prepared by mechanical disruption of tissues between frosted microscope slides in phosphate buffered saline (PBS) and cells were counted after RBC lysis using a Z2 Coulter Particle Count and Size Analyzer (Beckman Coulter). One ear was collected in 2 mL of RPMI for immune phenotyping and one ear was collected in 0.5 mL of RNAlater for subsequent gene expression analysis (see below). Ear cell suspensions were prepared by splitting ear into ventral and dorsal halves, followed by an enzymatic digestion for 90 min at 37 °C with 0.25 mg/mL Liberase-TL Research grade (Roche) in RPMI with 100 μg/mL DNase I (Sigma-Aldrich). Digestion was stopped by the addition of 3 mL of RPMI +10% FBS, the ears + media were transferred to gentleMACS C Tubes (Miltenyi Biotec), then cells were mechanically disrupted on a gentleMACS™ Dissocciator (Miltenyi Biotec). Following disruption, cells were passed through a 70 μm cell strainer to make a single cell suspension, washed with RPMI 10% FBS, then live cells were counted on a Cellometer using AO/PI (Nexcelom) in order to quantify cells. For staining, single cell suspensions were resuspended in staining buffer containing a-mouse CD16/32 antibody (Fc Block) (BD Biosciences) then incubated with a cocktail of fluorochrome-conjugated antibodies specific for mouse cell surface antigens. For LN and Spleen cells: α-IgE-FITC (R35–72), α-B220-V500 (RA3–6B2), α-CD8a-PE (53–6.7) (BD Biosciences, San Jose, CA), α-CD4-BV-605 (GK1.4) (BioLegend, San Diego, CA), α-CD11b-PerCP-Cyanine5.5 (M1/70), α-CD11c-eF-450 (N418), α-CD25 PE-eF-601 (PC61.5), α-CD86-APC (GL1), α-MHC II-AF700 (M5/14.15.2), and α-CD44-eFluor 780 (IM7) (eBioscience). For ear cells: α-CD45-BV605 (30-F11), α-CD3-V500 (500A2), α-Siglec-F-PE (E50–2440), α-Ly6G-FITC (1A8) (BD Biosciences), Lineage-PerCP-Cyanine5.5 (α-Ter-119 [TER-119], α-CD19 [eBio1D3], α-CD11b [M1/70], and α-CD11c [N418]), α-CD90.2-Super Bright 780 (30-H12), α-CD117-eFlour 450 (2B8), α-FcεRI-APC (MAR-1) (eBioscience), and α-F4/80-APC-Fire750 (BM8) (Biolegend). Cells were then washed, fixed in Cytofix buffer (BD Biosciences), resuspended in staining buffer, and events were collected on an LSR II flow cytometer (BD Biosciences), compensation controls were prepared with eBioscience UltraComp eBeads.Analysis was performed using FlowJo v10 software (TreeStar Inc., Ashland, OR). All cells were gated on single events prior to subsequent gating. The IgE+B220+ (IgE+ B-cells) population were analyzed as described by Manetz and Meade (1999). Gating for the spleen and LN phenotyping was performed as previously described (Shane et al., 2017). For the phenotyping of the ear, cellular populations were defined as follows: hematopoietic cells/leukocytes (CD45+), T cells (CD45+,SSClow,CD45+ Lin−,CD90+,CD3+), mast cells (CD45+,SSChi,CD45+,Lin−,ckit+ FcεRI+), neutrophils (CD45+, CD11b+ Ly-6G+, SiglecF−), eosinophils (CD45+, CD11b+ Ly-6Gint SiglecF+ SSChi), monocytes/macrophages (CD45+, Lin+, F4/80+, SSClow).
2.7. Gene expression analysis
Ears (1/mouse) were mechanically disrupted on a TissueLyser II in Buffer RLT (Qiagen). Total RNA was extracted using Qiagen's RNeasy mini spin column kits with DNase treatment on a QIAcube workstation. RNA concentrations and purity were analyzed on a NanoDrop spec-trophotomer (Thermo Fisher Scientific). The cDNA (1–2 μg) was prepared on an Eppendorf Mastercycler using Applied Biosystems' High Capacity Reverse Transcription kit. The cDNA was used as template for real-time PCR reactions containing TaqMan PCR Master Mix with gene-specific primers (Applied Biosystems) on a 7500 Real-Time PCR System. Relative fold gene expression changes (2−ΔΔCT) were determined compared to acetone controls and normalized for expression of housekeeping gene beta-actin (Actb). Genes that were evaluated include: Tslp (Mm01157588_m1), Il1b (Mm00434228_m1), Il6 (Mm00446190_m1), PPARα (Mm00440930_m1), Nfkb1 (Mm00476361_m1), Flg2 (Mm02744902_ g1), Lor (Mm01962659_s1), and Itgbl1 (Mm01200043_m1).
2.8. Statistical analyses
For analysis of the data generated from the described animal studies, the data were first tested for homogeneity using the Bartletťs Chi Square test. If homogeneous, a one-way analysis of variance (ANOVA) was conducted. If the ANOVA showed significance at p < 0.05 or less, the Dunnetťs Multiple Range t-test was used to compare treatment groups with the control group. Linear trend analysis was performed to determine if PFOA had exposure concentration-related effects for the specified endpoints. Statistical analysis was performed using Graph Pad Prism version 5.0 (San Diego, CA). Statistical significance is designated by *p ≤ 0.05 and **p ≤ 0.01.
3. Results
3.1. In vivo studies did not identify PFOA to be an allergic sensitizer or dermal irritant
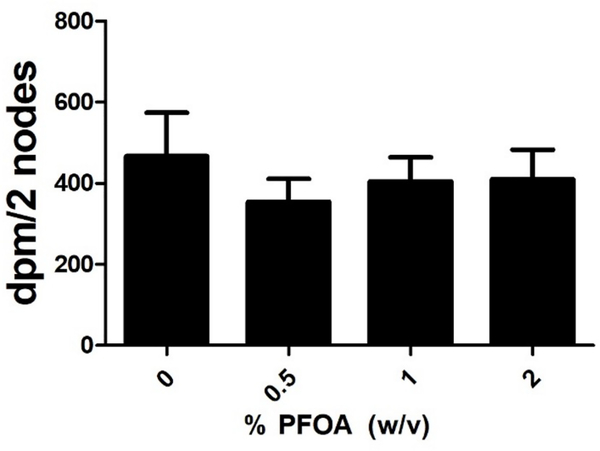
Dermal exposure to PFOA was found to be toxic (greater than 20% loss in body weigh) at concentrations greater than 2% (data not shown). For this reason, concentrations of PFOA up to 2% were tested in the subsequent studies. No ear swelling was observed in mice after dermal exposure to PFOA (data not shown). No increase in auricular DLN proliferation was identified after treatment with PFOA (Fig. 1). HCA (30%) was used as a positive control for these experiments and resulted in an average DPM/node of 4,704, indicating a 9.8 fold increase over vehicle (data not shown).
Fig. 1. Allergic sensitization potential after dermal exposure to PFOA.
Analysis of the allergic sensitization potential of PFOA using the LLNA. DPM represent [3H]-thymidine incorporation into draining lymph node cells of BALB/c mice following exposure to vehicle (0%) or concentration of PFOA. Bars represent mean (± SE) of 5 mice per group.
3.2. Dermal exposure to PFOA suppressed the splenic IgM response to SRBC
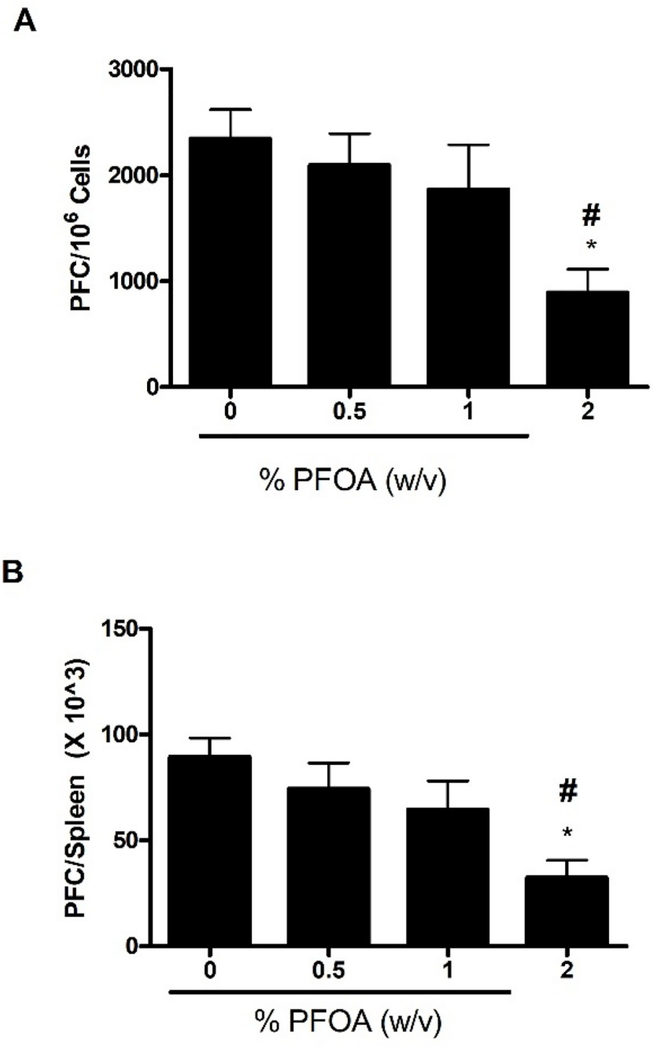
To evaluate if exposure to PFOA was immunosuppressive, the murine IgM response to SRBC was examined following a 4-day exposure to PFOA. Statistically significant reductions in the specific (PFC/106 cells) and PFC/spleen IgM antibody activity against SRBC were observed after exposure to PFOA (Fig. 2A and 2B). Exposure of mice to 2% PFOA resulted in a suppression of the values for PFC/106 cells and PFC/spleen (62 and 64%, respectively, vs. values for vehicle-treated mice); 1% PFOA resulted in suppressions of PFC/106 cells (20%) and PFC/spleen (27%); although it did not reach statistical significance (Fig. 2). However, there was a dose-responsive decrease in both PFC/106 cells and PFC/spleen (Linear Trend Test p ≤ 0.05). Mice exposed to cyclophosphamide had a significantly reduced specific spleen IgM response (70% reduction) and total IgM response (67% reduction) compared to levels noted in vehicle-treated controls (data not shown).
Fig. 2. Dermal PFOA exposure suppresses the spleen IgM response to SRBC.
Analysis of antibody producing spleen cells after a 4-day dermal exposure to PFOA suppressed the (A) specific and (B) total activity IgM response to SRBC. Bars represent mean fold-change (± SE) of 5 mice per group. Levels of statistical significance are denoted (*p < 0.05) as compared to acetone vehicle (0%). Linear Trend Test #p ≤ 0.05.
3.3. Dermal exposure to PFOA for 4 and 14 days results in significant alterations in organ weights
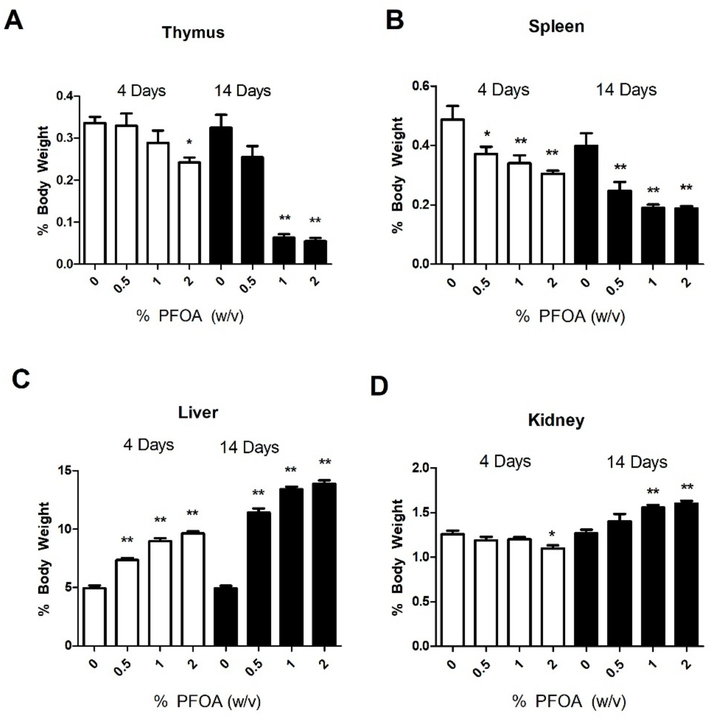
A statistically significant decrease in thymus and spleen weights (% body weight) was observed following exposure to PFOA (Fig. 3A and B). Thymus weight was significantly decreased by 4-days of 2% PFOA exposure and for 1 and 2% for 14-days of exposure (Fig. 3A). Spleen weight was significantly decreased for all concentrations of PFOA at 4 and 14-days of exposures (Fig. 3B). Liver weight (% body weight) was significantly increased for all concentrations of PFOA at 4 and 14-days of exposures (Fig. 3C). Kidney weight (% body weight) was significantly decreased at 4-days but only following 2% exposure (Fig. 3D). In contrast, by 14-days of PFOA exposure, kidney weight was increased for both the 1% and 2% exposure groups. Organ weights not corrected for total body weight are reported in Supplemental Table 1, where significant decreases in mass of the thymus (14 days, 0.5%–2%) and spleen (4 and 14 days; 0.5%–2%) were observed with significant increases in liver mass (4 and 14 days; 0.5%–2%). Exposure to 2% PFOA was terminated after 10 days due to a greater than 20% loss in body weight. No changes in body weight were observed following 4 days of PFOA exposure however, 14 days of exposure resulted in dose responsive decreases in body weight (Supplemental Fig. 1).
Fig. 3. Changes in organ weights after dermal exposure to PFOA.
Analysis of changes in thymus (A), spleen (B), liver (C), and kidney (D) weights following 4 and 14 days of PFOA exposure. Data is displayed as organ weight as % of body weight in order to normalize between different sizes of mice. Bars represent mean (± SE) of 5 mice per group. Levels of statistical significance are denoted (**p < 0.01 and * p < 0.05) as compared to acetone vehicle (0%).
3.4. Dermal exposure to PFOA for 4 and 14 days results in significant phenotypic changes in the spleen and DLN
Overall, the splenic cellularity was reduced, and reductions in cell numbers were observed after both 4 and 14 days of exposure to PFOA (0.5%, 1% and 2%) in all subsets of cells analyzed (Table 1), reflective of the reduction in total spleen mass (Fig. 3). However, certain subsets of cells were disproportionally reduced. Dermal exposure to PFOA for 4 days did not result in changes in the frequency of spleen CD4 T-cells, CD8 T-cells, B-cells, dendritic cells (DCs), or monocytes (Table 1). However, following 14 days of dermal PFOA exposure increases were observed in the frequency of CD4 T-cells (0.5%, 1%, and 2%) and CD8 T-cells (0.5%, 1%, and 2%) along with a significant decrease in the frequency of B-cells (1% and 2%) (Table 1). A dose dependent increase in monocytes (reaching statistical significance at the 2% PFOA concentration) was also observed. Phenotypic analysis of the DLN following 4 days of PFOA exposure resulted in a significant increase in the frequency of DCs (2%) and a decrease in the frequency of monocytes (1%) (Table 2). The significant increase in DCs was also observed following 14 days of exposure to 2% PFOA. However, no changes in the frequency of monocytes was observed following 14-days of PFOA exposure. The extended PFOA exposure also resulted in a decrease in the frequency of CD4 T-cells in the LN (1% and 2%) and a decrease in the frequency of CD8 T-cells (1% and 2%) in the DLN. Exposure to 2% PFOA for 4 days produced statistically significant increases in the absolute number of total cells, B-cells, CD4+ T-cells, CD8+ T-cells, DCs, and monocytes (Table 2). However, for the 14-day exposure statistically significant decreases were observed for total cells (1% and 2%), B-cells (1%), and CD4+ T-cells (1% and 2%).
Table 1.
Spleen phenotyping following dermal exposure to PFOA.
| Exposure Duration | 4 Days | 14 Days | ||||||
|---|---|---|---|---|---|---|---|---|
| PFOA (w/v) | 0% | 0.5% | 1% | 2% | 0% | 0.5% | 1% | 2% |
| Cellularity (× 107) | 9.48 ± 1.29 | 6.52 ± 0.40** | 4.54 ± 0.38** | 4.59 ± 0.47** | 8.85 ± 0.75 | 3.78 ± 0.62** | 0.86 ± 0.10** | 0.82 ± 0.08** |
| CD4+ (× 107) | 2.24 ± 1.24 | 1.67 ± 1.38** | 1.11 ± 0.85** | 1.19 ± 0.13** | 1.93 ± 0.17 | 1.14 ± 0.15** | 0.30 ± 0.02** | 0.27 ± 0.02** |
| CD4+ (%) | 23.70 ± 0.43 | 25.50 ± 0.53 | 24.72 ± 0.48 | 25.90 ± 1.22 | 21.82 ± 0.74 | 31.02 ± 1.69** | 36.50 ± 1.95** | 33.58 ± 1.00** |
| CD8+ (× 106) | 10.85 ± 0.57 | 7.62 ± 0.45** | 5.07 ± 0.44** | 5.32 ± 0.51** | 9.19 ± 0.80 | 5.22 ± 0.78** | 1.33 ± 0.11** | 1.28 ± 0.12** |
| CD8+ (%) | 11.48 ± 0.19 | 11.70 ± 0.28 | 11.16 ± 0.32 | 11.64 ± 0.34 | 10.41 ± 0.39 | 14.00 ± 0.42** | 15.84 ± 0.76** | 15.62 ± 0.57** |
| B-cells (× 107) | 3.50 ± 0.22 | 2.45 ± 0.14** | 1.69 ± 0.16** | 1.74 ± 0.19** | 3.45 ± 0.33 | 1.32 ± 0.24** | 0.27 ± 0.03** | 0.21 ± 0.03** |
| B-cells (%) | 36.94 ± 0.70 | 37.68 ± 0.73 | 37.14 ± 0.47 | 37.84 ± 0.51 | 38.82 ± 0.61 | 34.42 ± 1.22 | 25.72 ± 1.68** | 25.20 ± 1.73** |
| Dendritic Cells (× 105) | 9.98 ± 0.11 | 7.12 ± 0.15** | 5.30 ± 0.54** | 5.63 ± 0.54** | 7.80 ± 0.68 | 4.32 ± 0.64** | 0.76 ± 0.12** | 0.88 ± 0.10** |
| Dendritic Cells (%) | 1.06 ± 0.05 | 1.10 ± 0.05 | 1.16 ± 0.07 | 1.23 ± 0.04 | 0.88 ± 0.03 | 1.16 ± 0.06** | 0.86 ± 0.07 | 1.06 ± 0.04 |
| Monocytes (× 106) | 3.73 ± 0.31 | 2.53 ± 0.78** | 1.75 ± 0.17** | 1.72 ± 0.18** | 3.31 ± 0.35** | 1.15 ± 0.18** | 0.42 ± 0.07** | 0.50 ± 0.03** |
| Monocytes (%) | 3.92 ± 0.16 | 3.93 ± 0.20 | 3.84 ± 0.14 | 3.78 ± 0.23 | 3.71 ± 0.16 | 3.10 ± 0.18 | 4.97 ± 0.56 | 6.35 ± 0.62** |
Spleen immune phenotyping following 4 and 14 days of PFOA exposure. Numbers represent mean (± SE) of 5 mice per group. Levels of statistical significance are denoted (**p < 0.01 and * p < 0.05) as compared to acetone vehicle (0%).
Table 2.
Lymph node phenotyping following dermal exposure to PFOA.
| Exposure Duration | 4 Days | 14 Days | ||||||
|---|---|---|---|---|---|---|---|---|
| PFOA (w/v) | 0% | 0.5% | 1% | 2% | 0% | 0.5% | 1% | 2% |
| Cellularity (× 106) | 4.50 ± 0.85 | 5.11 ± 0.66 | 4.94 ± 0.53 | 9.02 ± 1.31* | 4.69 ± 0.65 | 3.73 ± 0.29 | 2.34 ± 0.23* | 2.86 ± 0.42** |
| CD4+ (× 106) | 2.34 ± 0.43 | 2.70 ± 0.35 | 2.60 ± 0.26 | 4.70 ± 0.68* | 2.57 ± 0.35 | 2.06 ± 0.19 | 1.09 ± 0.13** | 1.20 ± 0.19** |
| CD4+ (%) | 52.12 ± 0.93 | 52.94 ± 0.50 | 52.96 ± 0.65 | 52.30 ± 1.06 | 54.82 ± 0.96 | 55.10 ± 0.78 | 46.18 ± 1.55* | 41.90 ± 1.73** |
| CD8+ (× 106) | 1.09 ± 0.16 | 1.27 ± 0.16 | 1.18 ± 0.13 | 2.19 ± 0.31* | 1.11 ± 0.14 | 1.01 ± 0.61 | 0.73 ± 0.07 | 0.87 ± 0.01 |
| CD8+ (%) | 24.90 ± 1.07 | 24.98 ± 0.28 | 23.84 ± 0.53 | 24.36 ± 0.23 | 23.78 ± 0.63 | 27.36 ± 0.91 | 31.53 ± 1.39** | 30.76 ± 0.30** |
| B-cells (× 106) | 0.72 ± 0.17 | 0.82 ± 0.11 | 0.85 ± 0.10 | 1.44 ± 0.23* | 0.87 ± 0.14 | 0.53 ± 0.04 | 0.39 ± 0.01* | 0.57 ± 0.10 |
| B-cells (%) | 15.42 ± 1.23 | 16.00 ± 0.78 | 17.18 ± 0.85 | 15.92 ± 0.96 | 18.42 ± 1.17 | 14.32 ± 0.58* | 17.28 ± 1.41 | 19.64 ± 1.17 |
| Dendritic Cells (× 104) | 3.02 ± 0.82 | 3.43 ± 0.44 | 3.39 ± 0.41 | 9.24 ± 0.13** | 2.36 ± 0.27 | 2.04 ± 0.19 | 1.63 ± 0.29 | 2.14 ± 0.23 |
| Dendritic Cells (%) | 0.63 ± 0.08 | 0.67 ± 0.04 | 0.69 ± 0.04 | 1.03 ± 0.08** | 0.51 ± 0.03 | 0.55 ± 0.05 | 0.68 ± 0.06 | 0.77 ± 0.07** |
| Monocytes (× 105) | 2.25 ± 0.44 | 1.82 ± 0.18 | 1.66 ± 0.19 | 4.06 ± 0.71* | 1.78 ± 0.28 | 1.36 ± 0.15 | 1.03 ± 0.16 | 1.41 ± 0.27 |
| Monocytes (%) | 5.20 ± 0.91 | 3.68 ± 0.29 | 3.35 ± 0.08* | 4.43 ± 0.29 | 3.80 ± 0.38 | 3.71 ± 0.46 | 4.34 ± 0.47 | 4.87 ± 0.54** |
Lymph node immune phenotyping following 4 and 14 days of PFOA exposure. Numbers represent mean (± SE) of 5 mice per group. Levels of statistical significance are denoted (**p < 0.01 and * p < 0.05) as compared to acetone vehicle (0%).
3.5. Dermal PFOA exposure results in immunological changes in the skin
Dermal exposure to PFOA for 4 days did not result in changes in the frequency of CD45 cells, T-cells, mast cells, eosinophils, or monocytes in the ear tissue (Table 3). A statistically significant increase in the frequency of neutrophils was observed but only following 4 days of 2% PFOA exposure. Following 14 days of dermal PFOA exposure decreases were observed in the total number of cells (2%), frequency of CD45 cells (1% and 2%) and eosinophils (0.5%, 1%, 2%). Increases were observed in the frequency of T-cells (1%, and 2%) and mast cells (1%). A small but statistically significant decrease in cellularity was observed but only following 14 days of 2% PFOA (1.21 × 106 ± 1.03 × 105) compared to the acetone control (1.56 × 106 ± 8.5 × 104). Significant increases in absolute numbers of CD45, T-cells, neutrophils, and monocytes were observed but only following exposure to 2% PFOA for 4 days (Table 3). However, following 14 days of PFOA exposure decreases in CD45 cells (1% and 2%), eosinophils (0.5%, 1%, 2%) and monocytes (1% and 2%) were observed.
Table 3.
Skin phenotyping following dermal exposure to PFOA.
| Exposure Duration | 4 Days | 14 Days | ||||||
|---|---|---|---|---|---|---|---|---|
| PFOA (w/v) | 0% | 0.5% | 1% | 2% | 0% | 0.5% | 1% | 2% |
| Cellularity (× 106) | 2.82 ± 0.18 | 3.03 ± 0.15 | 2.86 ± 0.10 | 3.49 ± 0.35 | 1.55 ± 0.08 | 1.82 ± 0.09 | 1.41 ± 0.05 | 1.21 ± 0.01* |
| CD45+ (× 105) | 2.38 ± 0.17 | 2.71 ± 0.07 | 2.11 ± 0.17 | 3.54 ± 0.36** | 1.05 ± 0.07 | 1.15 ± 0.07 | 0.65 ± 0.04** | 0.60 ± 0.05** |
| CD45+ (%) | 8.48 ± 0.35 | 9.04 ± 0.55 | 7.34 ± 0.38 | 10.29 ± 1.00 | 6.81 ± 0.39 | 6.31 ± 0.25 | 4.62 ± 0.20** | 5.02 ± 0.43** |
| T-cells (× 104) | 0.91 ± 0.07 | 1.02 ± 0.14 | 0.85 ± 0.08 | 1.44 ± 0.79** | 0.48 ± 0.01 | 0.64 ± 0.06 | 0.41 ± 0.02 | 0.37 ± 0.06 |
| T-cells (%) | 3.84 ± 0.24 | 3.77 ± 0.50 | 4.04 ± 0.22 | 4.16 ± 0.25 | 4.60 ± 0.21 | 5.58 ± 0.44 | 6.36 ± 0.21 | 6.15 ± 0.54 |
| Mast cells (× 104) | 1.09 ± 0.98 | 1.32 ± 0.16 | 0.87 ± 0.05* | 1.62 ± 0.22 | 0.59 ± 0.79 | 0.54 ± 0.07 | 0.30 ± 0.01 | 0.38 ± 0.27 |
| Mast cells (%) | 4.57 ± 0.14 | 4.85 ± 0.51 | 4.20 ± 0.33 | 4.52 ± 0.27 | 5.52 ± 0.39 | 4.71 ± 0.38 | 4.61 ± 0.16* | 6.38 ± 0.24 |
| Neutrophils (× 103) | 1.41 ± 0.13 | 1.52 ± 0.19 | 1.22 ± 0.23 | 8.64 ± 1.97** | 0.69 ± 0.05 | 0.64 ± 0.05 | 0.81 ± 0.02 | 0.61 ± 0.01 |
| Neutrophils (%) | 0.59 ± 0.03 | 0.56 ± 0.06 | 0.56 ± 0.08 | 2.35 ± 0.47** | 0.65 ± 0.03 | 0.56 ± 0.03 | 1.19 ± 0.23 | 1.05 ± 0.22 |
| Monocytes (× 105) | 1.08 ± 0.03 | 1.25 ± 0.03 | 1.05 ± 0.07 | 1.47 ± 0.13* | 0.49 ± 0.03 | 0.56 ± 0.04 | 0.31 ± 0.02** | 0.30 ± 0.02** |
| Monocytes (%) | 45.94 ± 1.98 | 46.22 ± 1.13 | 49.86 ± 0.79 | 41.88 ± 1.18 | 47.02 ± 0.37 | 48.64 ± 1.12 | 48.22 ± 0.74 | 50.18 ± 0.92 |
| Eosinophils (× 104) | 1.16 ± 0.16 | 1.71 ± 0.12 | 0.86 ± 0.94 | 2.59 ± 0.62* | 0.67 ± 0.55 | 0.33 ± 0.56** | 0.05 ± 0.01** | 0.10 ± 0.03** |
| Eosinophils (%) | 4.82 ± 0.65 | 6.29 ± 0.36 | 4.18 ± 0.48 | 7.03 ± 1.25 | 6.33 ± 0.13 | 2.92 ± 0.47** | 0.81 ± 0.17** | 1.51 ± 0.46** |
Immune phenotyping of the skin following 4 and 14 days of PFOA exposure. Numbers represent mean (± SE) of 5 mice per group. Levels of statistical significance are denoted (**p < 0.01 and * p < 0.05) as compared to acetone vehicle (0%).
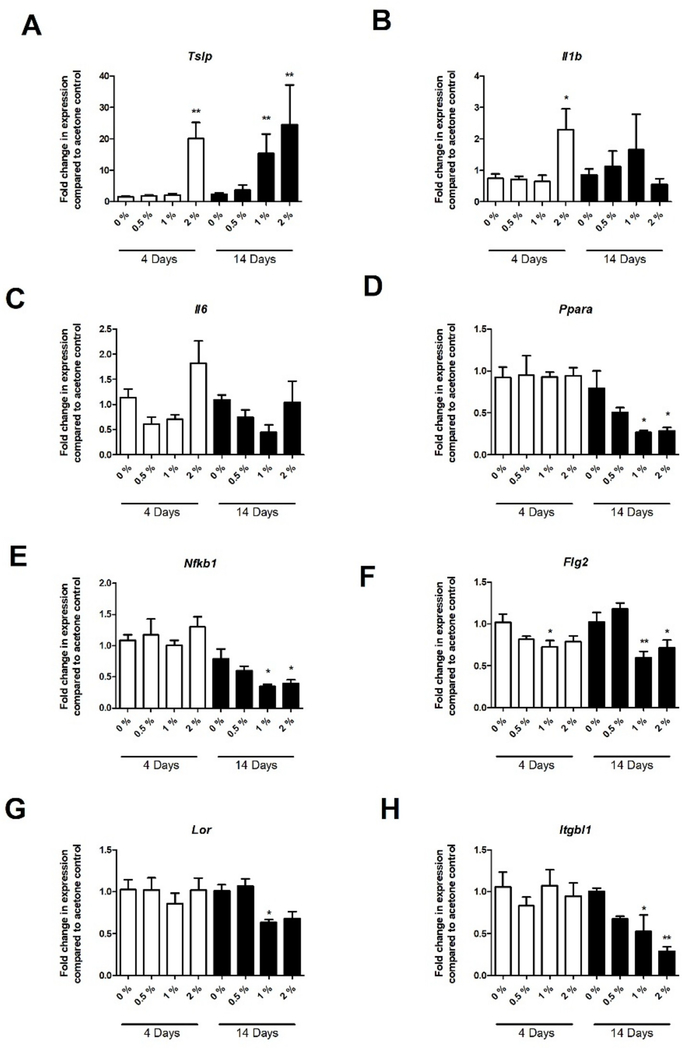
In an attempt to better define the mechanism of dermal immunotoxicity, select mRNA transcripts were evaluated in the skin following PFOA exposure. Increases in the Th2 skewing cytokine Tslp were observed at both PFOA exposure durations (Fig. 4A). A small but significant increase in the inflammatory cytokines Il1b and Il6 were observed but only following the 4 day 2% PFOA exposure (Fig. 4B and C). Interestingly, decreases in peroxisome proliferator-activated receptor alpha (PPARα) and nuclear factor kappa B (Nfkb1) expression were observed at all concentrations but only for the 14-day exposure duration (Fig. 4D and E). Expression in genes involved in skin barrier integrity were also evaluated. Decreases in filaggrin (Flg2), integrin subunit beta like 1 (Itgbl1) and loricrin (Lor) were observed, but only for the extended PFOA exposure duration (Fig. 4F–H).
Fig. 4. Skin gene expression following dermal exposure to PFOA.
Gene expression in the skin following 4 and 14 days of PFOA exposure. Changes in Tslp (A), Il-1beta (B), Il-6 (C), PPARα (D), Nfkb1 (E), Flg2 (F), Lor (G), and Itgbl1 (H) were evaluated. Bars represent mean (± SE) of 5 mice per group. Levels of statistical significance are denoted (**p < 0.01 and * p < 0.05) as compared to acetone vehicle (0%).
4. Discussion
A large number of workers in the United States are potentially exposed to chemicals that can be absorbed through the skin (Anderson et al., 2014). Since immune dysfunction can affect multiple organ systems, there is an increasing need to evaluate these chemicals and/or substances. A major role of the skin, as the largest organ in the body, is to serve as a barrier to protect from environmental and chemical insults. Disruption of barrier function may lead to inflammatory and immunological responses in the skin and other tissues (Hanel et al., 2013). In addition to providing protection from the outside environment, the skin is an extremely important player in immunological responses. The skin is an immunologically active organ that must maintain a delicate balance between pro-inflammatory and anti-inflammatory immune responses in order to react against pathogens and yet mitigate unnecessary tissue damage. In order to achieve this balance, the skin is highly integrated with a diverse milieu of regulatory and inflammatory immune cells and mediators that are unique to this tissue. In an attempt to fill some of the data gaps associated with dermal PFOA exposure-related health effects, the immunotoxicity of PFOA was evaluated using a murine model in the studies described here. It is important to note that the overall purpose of this paper is for hazard identification, as such the concentrations of PFOA used in this manuscript were chosen based on preliminary data generated in our laboratory which showed immunotoxic effects following dermal exposure. Dermal exposure to 0.5%–2% PFOA resulted in detectable levels of PFOA in the serum, with 4 d of dermal exposure to 1% PFOA resulting in serum levels of 188 ± 16 μg/mL (Franko et al., 2012). These experimental concentrations are approximately 15- to 30-fold higher than those measured in the serum of the highest occupationally exposed human populations, (Kudo and Kawashima, 2003). It should be noted that comparisons between species can be difficult to make as human exposures are often chronic in nature and PFAS are excreted/eliminated at different rates in humans (Ubel et al., 1980) and in different experimental animal systems/sexes (Vanden Heuvel et al., 1991). However, the serum PFOA levels occurring after dermal exposure are for the purposes of hazard identification and are comparable to other experimental animal studies in the literature based on oral exposure to PFOA via gavage (Dewitt et al., 2008).
Similar to what has been described in the literature following oral exposure, suppression of the IgM response to SRBC was observed following dermal exposure to this chemical (Fig. 2). Phenotypic analysis of the spleen showed decreased cellularity in addition to decreases in absolute number and frequency of B-cells, further supporting the suppressive effect (Table 1). The T-cell dependent antibody response is one of the most sensitive indicators of immune integrity because it relies on an organized immune response that is dependent on the functional capacity and cooperation of numerous cell types including B-cells, T-cells, and macrophages (Anderson et al., 2006). Additional evidence for suppression and immunotoxicity was evidenced by decreases in spleen and thymus weights along with increases in liver weights as early as 4 days following dermal PFOA exposure (Fig. 3). In the DLN, by 14 days of exposure, total cell numbers and the frequency of CD4+ subsets were significantly decreased (Table 2). While earlier studies identified immunotoxicity following a 4 day dermal PFOA exposure (Fairley et al., 2007a), recent findings in our laboratory suggest that exposure duration might also influence immunological response (Shane et al., 2017). Therefore, a 4 day and 14 day exposure duration were examined in the current study. While the exposure duration for the mice exposed to 2% PFOA had to be terminated due to the onset of overt toxicity (Supplemental Fig. 1), the majority of significant effects were also observed at lower concentrations that did not result in excessive loss in body weight.
In addition to other pathways, PFOA has been shown to trigger biological activity by activating the alpha isotype of peroxisome proliferator-activated receptors (PPARα), ligand-activated transcription factors that regulate gene expression (Li et al., 2017). Mechanistically, PPARα ligands block the NF-ĸB pathway and thereby modulate subsequent immune responses. Activation of PPARα modulates lipid and glucose homeostasis, cell proliferation and differentiation, and inflammation (DeWitt et al., 2009b). However, PPARα independent PFOA induced immunological effects have also been demonstrated in PPARα knockout animal models and it has been suggested PFOA induced immune suppression is mediated via a PPARα independent pathway most likely due to B-cell disruption (DeWitt et al., 2016). PPARα is expressed in many cutaneous immune cells types including macrophages, keratinocytes, and T-lymphocytes where it regulates inflammatory responses (Dubrac et al., 2011). In an attempt to better define the dermal PFOA immunotoxicity, PPARα expression was examined in the skin. Interestingly, PFOA did not increase expression of PPARα in the skin following dermal exposure at the time points evaluated but instead resulted in significant decreases following the 14 days of exposure (Fig. 4). However, expression of PPARα at early time points (less than 4 days of exposure) was not evaluated. Consistent with PPARα activation, expression of Nfkb1 was decreased by 14 days and no persistent signs of inflammation (evidenced by increases in Il6 and Il1b) were observed. However, a slight inflammatory response was observed after 4 days of exposure supported by increases in T-cell numbers in the DLN and skin, increases in eosinophil and neutrophil number and increases in inflammatory cytokines (Il1b and Il6) in the skin which completely resolved or decreased by 14 days (Tables 2 & 3 and Fig. 4).
While described as a PPARα agonist, no increases in PPARα were observed in the skin suggesting PFOA might not activate PPARα in the skin or that increases occurred at early time points which were not examined. Although this relationship has not been thoroughly investigated, differential expression and sensitivity of PFOA activation of PPARα has been reported (Abbott et al., 2012). The results from the present study demonstrate that PFOA is a non-irritating and non-sensitizing chemical as evidenced by the lack of increase in ear swelling and lymphocyte proliferation (Fig. 1). However, PFOA has also been shown to augment allergic disease in an animal model, but specific mechanisms have not been described (Fairley et al., 2007a). A lot of research has explored the therapeutic potential of PPARα agonists in inflammatory and allergic diseases and anti-inflammatory effects of PPARα activation have been reported in mouse models of irritant and allergic contact dermatitis (Furue et al., 2018). While PFOA might not induce direct activation of PPARα in the skin, there is involvement of this pathway as evidenced by significant reductions in expression. Research has demonstrated decreased expression of PPARα following permeability barrier abrogation and additional increases in Th2 cytokines in cultured normal human keratinocytes (Adachi et al., 2013). Due to the potential involvement of PPARα in skin barrier integrity, expression of the related mRNA transcripts Flg2, Lor, and Itgbl1 were evaluated. Filaggrin is a protein involved in epidermal barrier function and research has shown that its expression is decreased in individuals with skin barrier disorder and atopic dermatitis (Cabanillas et al., 2016). Loricrin is a protein important for skin barrier formation and integrity and has been shown to be reduced in the skin of individuals with atopic dermatitis (Kim et al., 2008). Itgbl1 has been suggested to play a role in intracellular adhesions (Symington et al., 1993). Consistent with these studies, results from the current study demonstrate a significant decrease in the expression of Flg2, Lor, and Itgbl1 following dermal PFOA exposure (Fig. 4). This suggests the potential involvement of PPARα in a cycle between barrier dysfunction and allergic inflammation. Although the findings support that PFOA might induce compromised skin integrity, no visual (irritation) or molecular indicators (inflammation) were identified. In addition, previous work has shown that PFOA solid is corrosive, but not at the 1% applied dose when evaluated in a cultured epidermis in vitro model (Franko et al., 2012).
In addition to allergic responses in the skin, inverse relationships have been identified between PPARα expression and asthma (Kobayashi et al., 2005). While direct PPARα agonists have been shown to reduce airway inflammation, decreases in PPARα expression may cause elevations in this response (Trifilieff et al., 2003) and PPARα agonists have been shown to reduce TSLP expression (Jung et al., 2018). Consistent with the decrease in PPARα expression, expression of the Th2 skewing cytokine, Tslp was elevated in PFOA exposed skin by 4 days of exposure and persisted throughout exposure duration (Fig. 4). Elevations in Tslp following dermal chemical exposure have also previously been shown by our laboratory to contribute to immune ad-juvancy (Anderson et al., 2013b; Marshall et al., 2015). Additional studies have associated PFOA exposure with allergic disease including previous work conducted in our laboratory that demonstrated dermal exposure to PFOA enhances allergen-specific responses in a murine model (Fairley et al., 2007b). Consistent with this hypothesis, a significantly greater prevalence of self-reported cases of chronic bronchitis and asthma were documented in individuals residing in a community with prolonged exposure to PFOA in their drinking water as compared to the general population (Anderson-Mahoney et al., 2008). A recent study also associated PFOA exposure with earlier onset of atopic dermatitis further supporting the association of PFOA exposure with allergic disease (Wen et al., 2019).
These are the first studies to evaluate the allergenic potential and immunotoxicity induced by dermal exposure to PFOA using a murine model. The findings suggest the potential immunological pathway include direct and/or indirect PPARα effects which might be a result of compromised skin barrier integrity. As human PPARα expression is significantly less than that of rodents, potential PPARα independence indicates that future research should explore mechanisms of action of PFOA and similar compounds, including PPARα-dependent and independent pathways (Chang et al., 2016; DeWitt et al., 2009b). Although significant data gaps still exist for the complete toxicological evaluation of this chemical, these results suggest that PFOA is an immunotoxic chemical following dermal exposure. Understanding the exposure routes relevant to PFOA toxicity will aid in establishing more effective guidelines for personal protective device usage and engineering controls that can help reduce exposure, as well as provide insight into potential mechanisms of action.
Supplementary Material
Acknowledgments
Funding
This work was supported by internal funds from the Health Effects Laboratory Division of the National Institute for Occupational Safety and Health
Abbreviations:
- ANOVA
analysis of variance
- DC
dendritic cell
- DLN
draining lymph nodes
- HCA
α-hexylcinnamaldehyde
- LLNA
local lymph node assay
- NF- ĸB
nuclear factor kappa B
- PFAS
per- and polyfluoroalkyl substance
- PFC
plaque forming cells
- PFOA
Perfluorooctanoic acid
- PPARα
alpha isotype of peroxisome proliferator-activated receptors
- SRBC
sheep red blood cell
- TSLP
thymic stromal lymphopoietin
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fct.2020.111114.
References
- Abbott BD, Wood CR, Watkins AM, Tatum-Gibbs K, Das KP, Lau C, 2012. Effects of perfluorooctanoic acid (PFOA) on expression of peroxisome proliferator-activated receptors (PPAR) and nuclear receptor-regulated genes in fetal and postnatal CD-1 mouse tissues. Reprod. Toxicol. 33 (4), 491–505. 10.1016/j.reprotox.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Adachi Y, Hatano Y, Sakai T, Fujiwara S, 2013. Expressions of peroxisome proliferator-activated receptors (PPARs) are directly influenced by permeability barrier abrogation and inflammatory cytokines and depressed PPARalpha modulates expressions of chemokines and epidermal differentiation-related molecules in keratinocytes. Exp. Dermatol. 22 (9), 606–608. 10.1111/exd.12208. [DOI] [PubMed] [Google Scholar]
- Anderson SE, Munson AE, Meade BJ, 2006. Analysis of Immunotoxicity by Enumeration of Antibody-Producing B cells.. Curr. Protoc. Toxicol. 29 (1). https://doi. org/10.1002/0471140856.tx1811s29. [DOI] [PubMed] [Google Scholar]
- Anderson-Mahoney P, Kotlerman J, Takhar H, Gray D, Dahlgren J, 2008. Self-reported health effects among community residents exposed to perfluorooctanoate. New Solut. 18 (2), 129–143. [DOI] [PubMed] [Google Scholar]
- Anderson SE, Franko J, Anderson KL, Munson AE, Lukomska E, Meade BJ, 2013a. Immunotoxicity and allergic potential induced by topical application of dimethyl carbonate (DMC) in a murine model. J. Immunotoxicol. 10 (1), 59–66. 10.3109/1547691x.2012.691124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SE, Franko J, Kashon ML, Anderson KL, Hubbs AF, Lukomska E, Meade BJ, 2013b. Exposure to triclosan augments the allergic response to ovalbumin in a mouse model of asthma. Toxicol. Sci.: Off. J. Soc. Toxicol. 132 (1), 96–106. 10.1093/toxsci/kfs328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SE, Meade BJ, 2014. Potential health effects associated with dermal exposure to occupational chemicals. Environ. Health Insights 8 (Suppl. 1), 51–62. 10.4137/ehi.s15258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR, 2018. DRAFT toxicological profile for perfluoroalkyls. Available at: https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf Accessed.
- Barry V, Winquist A, Steenland K, 2013. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ. Health Perspect. 121 (11–12), 1313–1318. 10.1289/ehp.1306615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley TH, White K, Honigfort P, Twaroski ML, Neches R, Walker RA, 2005. Perfluorochemicals: potential sources of and migration from food packaging. Food Addit. Contam. 22 (10), 1023–1031. [DOI] [PubMed] [Google Scholar]
- Betts KS, 2007. Perfluoroalkyl acids: what is the evidence telling us? Environ. Health Perspect. 115 (5), A250–A256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabanillas B, Novak N, 2016. Atopic dermatitis and filaggrin. Curr. Opin. Immunol. 42, 1–8. 10.1016/j.coi.2016.05.002. [DOI] [PubMed] [Google Scholar]
- Chang ET, Adami HO, Boffetta P, Wedner HJ, Mandel JS, 2016. A critical review of perfluorooctanoate and perfluorooctanesulfonate exposure and immunological health conditions in humans. Crit. Rev. Toxicol. 46 (4), 279–331. 10.3109/10408444.2015.1122573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt JC, Copeland CB, Luebke RW, 2009a. Suppression of humoral immunity by perfluorooctanoic acid is independent of elevated serum corticosterone concentration in mice. Toxicol. Sci.: Off. J. Soc. Toxicol. 109 (1), 106–112. 10.1093/toxsci/kfp040. (Research Support, Non-U.S. Govť, Research Support, U.S. Govť, Non-P.H.S.). [DOI] [PubMed] [Google Scholar]
- Dewitt JC, Copeland CB, Strynar MJ, Luebke RW, 2008. Perfluorooctanoic acid-induced immunomodulation in adult C57BL/6J or C57BL/6N female mice. Environ. Health Perspect. 116 (5), 644–650. 10.1289/ehp.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt JC, Shnyra A, Badr MZ, Loveless SE, Hoban D, Frame SR, Cunard R, Anderson SE, Meade BJ, Peden-Adams MM, et al. , 2009b. Immunotoxicity of perfluorooctanoic acid and perfluorooctane sulfonate and the role of peroxisome proliferator-activated receptor alpha. Crit. Rev. Toxicol. 39 (1), 76–94. 10.1080/10408440802209804. [DOI] [PubMed] [Google Scholar]
- DeWitt JC, Williams WC, Creech NJ, Luebke RW, 2016. Suppression of antigen-specific antibody responses in mice exposed to perfluorooctanoic acid: role of PPARalpha and T- and B-cell targeting. J. Immunotoxicol. 13 (1), 38–45. https://doi. org/10.3109/1547691x.2014.996682. [DOI] [PubMed] [Google Scholar]
- Dubrac S, Schmuth M, 2011. PPAR-alpha in cutaneous inflammation. Derm. Endocrinol. 3 (1), 23–26. 10.4161/derm.3.1.14615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett EA, Shofer FS, Zhang H, Freeman D, Desai C, Shaw LM, 2006a. Community exposure to perfluorooctanoate: relationships between serum concentrations and exposure sources. J. Occup. Environ. Med. 48 (8), 759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett EA, Zhang H, Shofer FS, Freeman D, Rodway NV, Desai C, Shaw LM, 2006b. Community exposure to perfluorooctanoate: relationships between serum levels and certain health parameters. J. Occup. Environ. Med. 48 (8), 771–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA, U.S, 2002. Office of Pollution Prevention and Toxics, Risk Assessment Division, Revised Draft Hazard Assessment of Perfluorooctanoic Acid and its Salts.
- EPA, U. S, 2019. USEPA Draft interim recommendations to address groundwater contaminated with Perfluorooctanoic Acid and Perfluorooctane Sulfonate.
- Fairley KJ, Purdy R, Kearns S, Anderson SE, Meade B, 2007a. Exposure to the immunosuppressant, perfluorooctanoic acid, enhances the murine IgE and airway hyperreactivity response to ovalbumin. Toxicol. Sci.: Off. J. Soc. Toxicol. 97 (2), 375–383. 10.1093/toxsci/kfm053. [DOI] [PubMed] [Google Scholar]
- Fairley KJ, Purdy R, Kearns S, Anderson SE, Meade BJ, 2007b. Exposure to the immunosuppressant, perfluorooctanoic acid, enhances the murine IgE and airway hyperreactivity response to ovalbumin. Toxicol. Sci.: Off. J. Soc. Toxicol. 97 (2), 375–383. 10.1093/toxsci/kfm053. [DOI] [PubMed] [Google Scholar]
- Franko J, Meade BJ, Frasch HF, Barbero AM, Anderson SE, 2012. Dermal penetration potential of perfluorooctanoic acid (PFOA) in human and mouse skin. J. Toxicol. Environ. Health Part A 75 (1), 50–62. 10.1080/15287394.2011.615108. [DOI] [PubMed] [Google Scholar]
- Frisbee SJ, Brooks AP Jr., Maher A, Flensborg P, Arnold S, Fletcher T, Steenland K, Shankar A, Knox SS, Pollard C, et al. , 2009. The C8 health project: design, methods, and participants. Environ. Health Perspect. 117 (12), 1873–1882. 10.1289/ehp.0800379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furue K, Mitoma C, Tsuji G, Furue M, 2018. Protective role of peroxisome proliferator-activated receptor alpha agonists in skin barrier and inflammation. Immunobiology 223 (3), 327–330. 10.1016/j.imbio.2017.10.047. [DOI] [PubMed] [Google Scholar]
- Giesy JP, Kannan K, 2001. Global distribution of perfluorooctane sulfonate in wildlife. Environ. Sci. Technol. 35 (7), 1339–1342. [DOI] [PubMed] [Google Scholar]
- Gilliland FD, Mandel JS, 1996. Serum perfluorooctanoic acid and hepatic enzymes, lipoproteins, and cholesterol: a study of occupationally exposed men. Am. J. Ind. Med. 29 (5), 560–568. [DOI] [PubMed] [Google Scholar]
- Hanel KH, Cornelissen C, Luscher B, Baron JM, 2013. Cytokines and the skin barrier. Int. J. Mol. Sci. 14 (4), 6720–6745. 10.3390/ijms14046720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick RL, Buckholz J, Biro FM, Calafat AM, Ye X, Xie C, Pinney SM, 2017. Polyfluoroalkyl substance exposure in the mid-Ohio river valley, 1991–2012. Environ. Pollut. 228, 50–60. 10.1016/j.envpol.2017.04.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITRC ITRC, 2017. History and Use of Per- and Polyfluoroalkyl Substances (PFAS).
- Jerne NK, Nordin AK, 1963. Plaque Formation in Agar by Single Antibody-Producing Cells. Science 140 (3565), 405 10.1126/science.140.3565.405. [DOI] [PubMed] [Google Scholar]
- Jung Y, Kim JC, Park NJ, Bong SK, Lee S, Jegal H, Jin LT, Kim SM, Kim YK, Kim SN, 2018. Eupatilin, an activator of PPARalpha, inhibits the development of oxazolone-induced atopic dermatitis symptoms in Balb/c mice. Biochem. Biophys. Res. Commun. 496 (2), 508–514. 10.1016/j.bbrc.2018.01.098. [DOI] [PubMed] [Google Scholar]
- Kannan K, Choi JW, Iseki N, Senthilkumar K, Kim DH, Giesy JP, 2002a. Concentrations of perfluorinated acids in livers of birds from Japan and Korea. Chemosphere 49 (3), 225–231. [DOI] [PubMed] [Google Scholar]
- Kannan K, Corsolini S, Falandysz J, Oehme G, Focardi S, Giesy JP, 2002b. Perfluorooctanesulfonate and related fluorinated hydrocarbons in marine mammals, fishes, and birds from coasts of the Baltic and the Mediterranean Seas. Environ. Sci. Technol. 36 (15), 3210–3216. [DOI] [PubMed] [Google Scholar]
- Kannan K, Franson JC, Bowerman WW, Hansen KJ, Jones PD, Giesy JP, 2001. Perfluorooctane sulfonate in fish-eating water birds including bald eagles and albatrosses. Environ. Sci. Technol. 35 (15), 3065–3070. [DOI] [PubMed] [Google Scholar]
- Kannan K, Newsted J, Halbrook RS, Giesy JP, 2002c. Perfluorooctanesulfonate and related fluorinated hydrocarbons in mink and river otters from the United States. Environ. Sci. Technol. 36 (12), 2566–2571. [DOI] [PubMed] [Google Scholar]
- Kennedy GL Jr., Butenhoff JL, Olsen GW, O'Connor JC, Seacat AM, Perkins RG, Biegel LB, Murphy SR, Farrar DG, 2004. The toxicology of perfluorooctanoate. Crit. Rev. Toxicol. 34 (4), 351–384. [DOI] [PubMed] [Google Scholar]
- Kim BE, Leung DY, Boguniewicz M, Howell MD, 2008. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin. Immunol. 126 (3), 332–337. 10.1016/j.clim.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink KJ, Meade BJ, 2003. Dermal exposure to 3-amino-5-mercapto-1,2,4-triazole (AMT) induces sensitization and airway hyperreactivity in BALB/c mice. Toxicol. Sci.: Off. J. Soc. Toxicol. 75 (1), 89–98. 10.1093/toxsci/kfg171. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Thomassen MJ, Rambasek T, Bonfield TL, Raychaudhuri B, Malur A, Winkler AR, Barna BP, Goldman SJ, Kavuru MS, 2005. An inverse relationship between peroxisome proliferator-activated receptor gamma and allergic airway inflammation in an allergen challenge model. Ann. Allergy Asthma Immunol.: Off. Publ. Am. Coll. Allergy Asthma Immunol. 95 (5), 468–473. 10.1016/s1081-1206(10)61173-8. [DOI] [PubMed] [Google Scholar]
- Kubwabo C, Stewart B, Zhu J, Marro L, 2005. Occurrence of perfluorosulfonates and other perfluorochemicals in dust from selected homes in the city of Ottawa, Canada. J. Environ. Monit. 7 (11), 1074–1078. [DOI] [PubMed] [Google Scholar]
- Kudo N, Kawashima Y, 2003. Toxicity and toxicokinetics of perfluorooctanoic acid in humans and animals. J. Toxicol. Sci. 28 (2), 49–57. [DOI] [PubMed] [Google Scholar]
- Li K, Gao P, Xiang P, Zhang X, Cui X, Ma LQ, 2017. Molecular mechanisms of PFOA-induced toxicity in animals and humans: implications for health risks. Environ. Int. 99, 43–54. 10.1016/j.envint.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Luster MI, Portier C, Pait DG, White KL Jr., Gennings C, Munson AE, Rosenthal GJ, 1992. Risk assessment in immunotoxicology. I. Sensitivity and predictability of immune tests. Fundam. Appl. Toxicol. 18 (2), 200–210. 10.1016/0272-0590(92)90047-l. [DOI] [PubMed] [Google Scholar]
- Manetz TS, Meade BJ, 1999. Development of a flow cytometry assay for the identification and differentiation of chemicals with the potential to elicit irritation, IgE-mediated, or T cell-mediated hypersensitivity responses. Toxicological sciences: an official journal of the Society of Toxicology 48 (2), 206–217. [DOI] [PubMed] [Google Scholar]
- Marshall NB, Lukomska E, Long CM, Kashon ML, Sharpnack DD, Nayak AP, Anderson KL, Jean Meade B, Anderson SE, 2015. Triclosan induces thymic stromal lymphopoietin in skin promoting Th2 allergic responses. Toxicol. Sci.: Off. J. Soc. Toxicol. 147 (1), 127–139. 10.1093/toxsci/kfv113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata H, Kannan K, Nasu T, Cho HS, Sinclair E, Takemurai A, 2006. Perfluorinated contaminants in sediments and aquatic organisms collected from shallow water and tidal flat areas of the Ariake Sea, Japan: environmental fate of perfluorooctane sulfonate in aquatic ecosystems. Environ. Sci. Technol. 40 (16), 4916–4921. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Burlew MM, Mandel JH, 2003. Epidemiologic assessment of worker serum perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) concentrations and medical surveillance examinations. J. Occup. Environ. Med. 45 (3), 260–270. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR, 2007. Half-life of serum elimination of perfluorooctanesulfonate,per-fluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 115 (9), 1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Gilliland FD, Burlew MM, Burris JM, Mandel JS, Mandel JH, 1998. An epidemiologic investigation of reproductive hormones in men with occupational exposure to perfluorooctanoic acid. J. Occup. Environ. Med. 40 (7), 614–622. [DOI] [PubMed] [Google Scholar]
- Shane HL, Lukomska E, Stefaniak AB, Anderson SE, 2017. Divergent hypersensitivity responses following topical application of the quaternary ammonium compound, didecyldimethylammonium bromide. J. Immunotoxicol. 14 (1), 204–214. 10.1080/1547691X.2017.1397826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkov AA, Wallace KB, 2002. Structural determinants of fluorochemical-induced mitochondrial dysfunction. Toxicol. Sci. 66 (2), 244–252. [DOI] [PubMed] [Google Scholar]
- Symington BE, Takada Y, Carter WG, 1993. Interaction of integrins alpha 3 beta 1 and alpha 2 beta 1: potential role in keratinocyte intercellular adhesion. J. Cell Biol. 120 (2), 523–535. 10.1083/jcb.120.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilieff A, Bench A, Hanley M, Bayley D, Campbell E, Whittaker P, 2003. PPAR-alpha and -gamma but not -delta agonists inhibit airway inflammation in a murine model of asthma: in vitro evidence for an NF-kappaB-independent effect. Br. J. Pharmacol. 139 (1), 163–171. 10.1038/sj.bjp.0705232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubel FA, Sorenson SD, Roach DE, 1980. Health status of plant workers exposed to fluorochemicals–a preliminary report. Am. Ind. Hyg. Assoc. J. 41 (8), 584–589. 10.1080/15298668091425310. [DOI] [PubMed] [Google Scholar]
- Vanden Heuvel JP, Kuslikis BI, Van Rafelghem MJ, Peterson RE, 1991. Tissue distribution, metabolism, and elimination of perfluorooctanoic acid in male and female rats. J. Biochem. Toxicol. 6 (2), 83–92. 10.1002/jbt.2570060202. [DOI] [PubMed] [Google Scholar]
- Wen HJ, Wang SL, Chuang YC, Chen PC, Guo YL, 2019. Prenatal perfluorooctanoic acid exposure is associated with early onset atopic dermatitis in 5-year-old children. Chemosphere 231, 25–31. 10.1016/j.chemosphere.2019.05.100. [DOI] [PubMed] [Google Scholar]
- Woolhiser MR, Munson AE, Meade BJ, 2000. Comparison of mouse strains using the local lymph node assay. Toxicology 146 (2–3), 221–227. [DOI] [PubMed] [Google Scholar]
- Yang Q, Abedi-Valugerdi M, Xie Y, Zhao XY, Moller G, Nelson BD, DePierre JW, 2002. Potent suppression of the adaptive immune response in mice upon dietary exposure to the potent peroxisome proliferator, perfluorooctanoic acid. Int. Immunopharmacol. 2 (2–3), 389–397. [DOI] [PubMed] [Google Scholar]
- Yang Q, Xie Y, Eriksson AM, Nelson BD, DePierre JW, 2001. Further evidence for the involvement of inhibition of cell proliferation and development in thymic and splenic atrophy induced by the peroxisome proliferator perfluoroctanoic acid in mice. Biochem. Pharmacol. 62 (8), 1133–1140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.