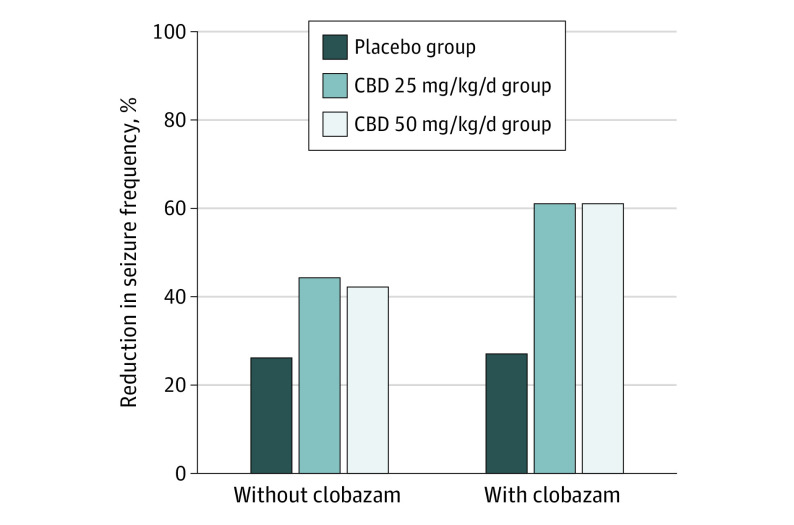
Figure 3. Change From Baseline in Frequency of Primary–End-Point Seizures During the Treatment Period in Patients Taking Cannabidiol Without Clobazam and With Clobazam.
Negative binomial regression was used to compare seizure frequency between the cannabidiol groups with the placebo group. The treatment period (16 weeks) constituted the titration and maintenance phases. The estimated ratio of least squares means for treatment period to baseline period was used to evaluate the reduction in seizure frequency. The primary–end-point seizures are all countable focal motor seizures without impairment of awareness, focal seizures with impairment of awareness, focal seizures evolving to bilateral motor seizures, and generalized seizures (tonic-clonic, tonic, clonic, or atonic). The percentage reduction from placebo was 24.7% (95% CI, 3.7%-41.1%) between placebo and cannabidiol 25 mg/kg/day (CBD25) groups and 22.0% (95% CI, −0.1% to 39.1%) between the placebo and cannabidiol 50 mg/kg/day (CBD50) groups among patients not taking clobazam and 46.6% (95% CI, 20.0%-64.4%) between the placebo and CBD25 groups and 46.6% (95% CI, 21.1%-63.9%) between the placebo and CBD50 groups among patients taking clobazam.