Abstract
Sulfonamides have played a defining role in the history of drug development and continue to be prevalent today. In particular, primary sulfonamides are common in marketed drugs. Here we describe the direct synthesis of these valuable compounds from organometallic reagents and a novel sulfinylamine reagent, t-BuONSO. A variety of (hetero)aryl and alkyl Grignard and organolithium reagents perform well in the reaction, providing primary sulfonamides in good to excellent yields in a convenient one-step process.
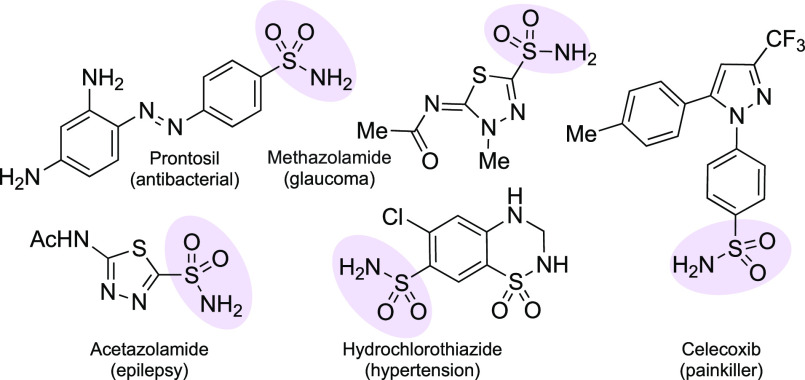
Nature has largely ignored sulfonamides when designing natural products;1 however, humans have taken advantage of their high stability, favorable physicochemical properties, and three-dimensional shape, in a rich variety of medicines since the advent of modern antibiotics. Among these, primary sulfonamides have featured prominently. The first sulfonamide drug, the antibacterial Prontosil,2 contains an aryl-SO2NH2 unit (Figure 1). They have also found use in treatments for epilepsy (Acetazolamide),3 high blood pressure (Hydrochlorothiazide),4 arthritis (Celecoxib),5 and glaucoma (Methazolamide).6 They remain popular to this day; sulfonamides are present as active pharmaceutical ingredients (APIs) in 16 out of 200 (8%) of the best-selling small molecule drugs of 2018.7
Figure 1.
Primary sulfonamide-containing drugs.
Primary sulfonamides have also found numerous applications in synthetic chemistry. Most commonly, they can be alkylated, acylated, or arylated to produce other sulfonamides.8 Notably, they are often the precursors to sulfonylureas, commonly used in diabetes medication9 and as herbicides,10 by coupling with isocyanates. Their combination with hypervalent iodine reagents enables relatively mild access to sulfonyl nitrene-type species. These intermediates have been exploited for the synthesis of amine derivatives by C–H insertion and aziridination, along with many other applications.11 An NHC-catalyzed deamination of primary sulfonamides to sulfinates has recently been developed by chemists at Merck, allowing them to act as precursors to sulfones, sulfonic acids, and other sulfonamides,12 as well as enabling isotopic labeling.13 The Cornella laboratory has recently reported methods for the conversion of primary sulfonamides to the corresponding sulfonyl chlorides and fluorides by activation with pyrylium salts.14 There are also examples of their use as directing groups15 for C–H functionalization.16 In the past few years, some notable functionalizations which expand the utility of primary sulfonamides have also appeared. These include Knowles’ proton-coupled electron transfer process to generate sulfonamidyl radicals under mild photoredox conditions, which can then add in an anti-Markovnikov fashion to alkenes,17 Stradiotto’s nickel-catalyzed cross-coupling of sulfonamides with (hetero)aryl chlorides,18 and MacMillan’s Ir/Ni photocatalytic coupling of sulfonamides with (hetero)aryl halides.19 A two-step nickel-catalyzed enantioselective reductive sulfonamidation of ketones20 and the first reported application of sulfonamides in the Petasis reaction21 were also recently disclosed.
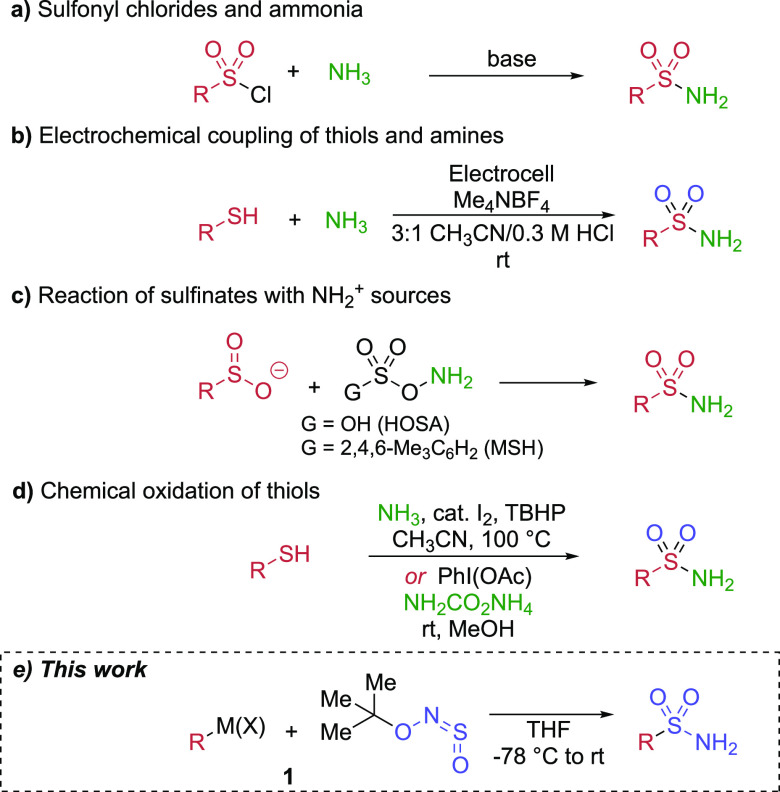
The classical synthesis of primary sulfonamides involves the reaction of activated sulfonyl electrophiles, usually sulfonyl chlorides, with ammonia, or an ammonia surrogate with a subsequent deprotection step (Scheme 1a). Although this reaction is still widely used where the appropriate sulfonyl chloride is easily available, it has some notable drawbacks. Sulfonyl chlorides are moisture-sensitive and are not always available due to limitations in both functional group tolerance and available substitution patterns inherent in their synthesis via harshly acidic and oxidizing chlorosulfonation conditions.22 Furthermore, the handling of gaseous ammonia can be challenging, while the use of solid or liquid ammonia surrogates necessarily leads to losses in atom and step economy. For these reasons, the development of alternative methods for sulfonamide synthesis in general, and primary sulfonamide synthesis in particular, has received much attention in recent years.
Scheme 1. Common Methods to Prepare Primary Sulfonamides: (a) Reaction of Sulfonyl Chlorides with Ammonia or Ammonia Surrogates; (b) Noël’s Electrochemical Approach; (c) Reaction of Sulfinates with NH2+ Sources; (d) Chemical Oxidation–Amination of Thiols; (e) Our Alkyl/Aryl Halide Based Approach.
Two recent papers have redefined the state of the art of sulfonamide synthesis. A copper-catalyzed direct synthesis of sulfonamides23 from the SO2 surrogate DABSO,24 boronic acids, and amines by our laboratory showed broad scope and functional group tolerance, but failed when ammonia was used. An elegant electrochemical synthesis of sulfonamides using thiols and amines from the Noël group25 did succeed in using ammonia (Scheme 1b). However, only one example was shown on a simple aryl scaffold, and electrochemistry has not yet been widely adopted in academic synthetic chemistry laboratories. The use of thiols as starting materials can also be problematic due to their malodorous nature and tendency to oxidize in air to form disulfides. Primary sulfonamides may also be prepared from sulfinate salts by reaction with an electrophilic nitrogen source such as O-mesitylenesulfonylhydroxylamine (MSH) or hydroxylamine-O-sulfonic acid (HOSA, Scheme 1c).26 This strategy is limited by the explosive risk of such reagents.27 Sulfinate salts can also undergo halogenation followed by the addition of an ammonia source.28 The low commercial availability of sulfinate salts is an issue, although new methods have further expanded access to these compounds, including by C–H activation (via thianthrenium salts and Pd catalysis)29 and using inexpensive nickel catalysts with DABSO and boronic acids.30 Useful oxidative syntheses of primary sulfonamides from thiols have also been developed,31 notably including a recent paper by Bull using iodobenzene diacetate and ammonium carbonate as an ammonia equivalent (Scheme 1d).32 Disadvantages of these methods include the use of thiols and lack of tolerance of some functional groups such as amines and thioethers to strong oxidants. Considering all these factors, a bespoke approach to primary sulfonamides starting from widely available alkyl and aryl halides would likely be welcomed by the synthetic community; the work reported in this Letter describes such an approach (Scheme 1e).
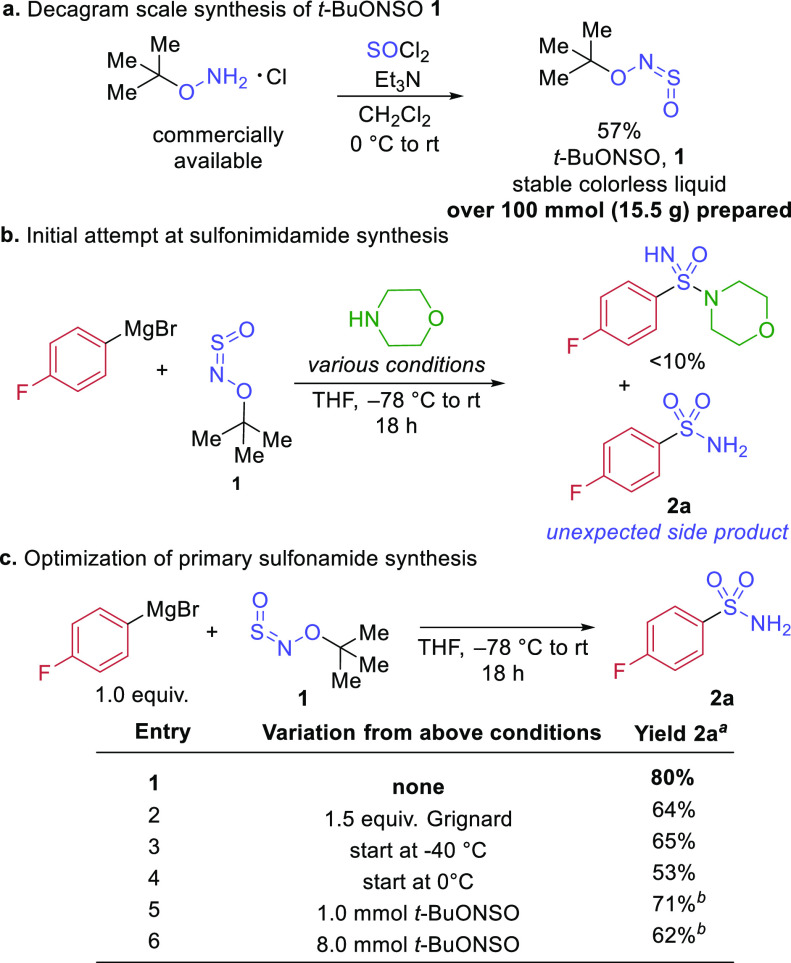
Our group has pioneered the use of sulfinylamine33 reagents (R(O)–N=S=O) for the preparation of synthetically and medicinally valuable high oxidation state sulfur compounds. Using organometallic nucleophiles generally derived from alkyl and aryl bromides, such as Grignard and organolithium reagents, we have designed one-pot syntheses of sulfonimidamides,34 sulfilimines (precursors to sulfondiimines),35 and sulfoximines.36 During our investigation into the synthesis of sulfoximines we developed a new class of sulfinylamines, N-sulfinyl-O-arylhydroxylamines, containing a cleavable N–O bond. When reacted with organometallic reagents at −78 °C, these compounds form highly electrophilic sulfinyl nitrenes;37 these reactive intermediates could then be reacted with a second carbon nucleophile, or amine, to give sulfoximines or sulfonimidamides, respectively. Our initial intention at the outset of this project was to develop a variant of this reaction which could be performed at noncryogenic temperatures. We therefore set out to design a reagent with a stronger N–O bond, reasoning that this would raise the barrier to N–O cleavage. We decided that replacing the aryl group on oxygen with an electron-releasing tert-butyl group would be optimal. The synthesis of this reagent, N-sulfinyl-O-(tert-butyl)hydroxylamine (t-BuONSO, 1), was conveniently achieved in one step using commercially available O-tert-butylhydroxylamine hydrochloride, thionyl chloride, and triethylamine, with a simple distillation (under reduced pressure) delivering the pure reagent 1 (Scheme 2a). The reaction was scalable and could be performed on 200 mmol scale to afford 15 g of t-BuONSO, as a stable, colorless nonviscous liquid.38
Scheme 2. Synthesis of t-BuONSO, 1, and Initial Reaction with Amine Leading to Reaction Discovery and Optimization.
Yield determined by 19F NMR spectroscopy. 0.3 mmol of t-BuONSO.
Isolated yields.
When we reacted t-BuONSO 1 with the commercially available Grignard reagent 4-fluorophenylmagnesium bromide and morpholine, in sequence at −78 °C, our standard reaction conditions for the preparation of sulfonimidamides using our original BiPhONSO reagent, we were frustrated to observe only 10% of the sulfonimidamide product in the crude reaction mixture (Scheme 2b). Similar reactions using two organometallic reagents as nucleophiles did not result in appreciable sulfoximine formation. Curiously, precipitation of a white solid was observed in both reactions when deuterated chloroform was added to the crude sample after aqueous workup. The solid did, however, dissolve in deuterated acetone, and we were surprised to find the 1H NMR spectra matched that of the primary sulfonamide 2a. Indeed, when the reaction was performed without the addition of a second nucleophile, product 2a was isolated in 80% yield. In the event, changing the structure of the sulfinylamine reagent did not result in different conditions for our previous reaction, but instead enabled a new, unusual primary sulfonamide synthesis. Increases in temperature and equivalents of Grignard reagent resulted in lower yields, confirming −78 °C and 1 equiv of the organometallic reagent as optimal (Scheme 2c). Importantly, the reactions could also be performed on preparative scale. For example, a reaction using 1 mmol of t-BuONSO delivered sulfonamide 2a in 71% yield (862 mg). A reaction using 1.098 g of t-BuONSO (8.0 mmol) provided sulfonamide 2a in 62% yield.
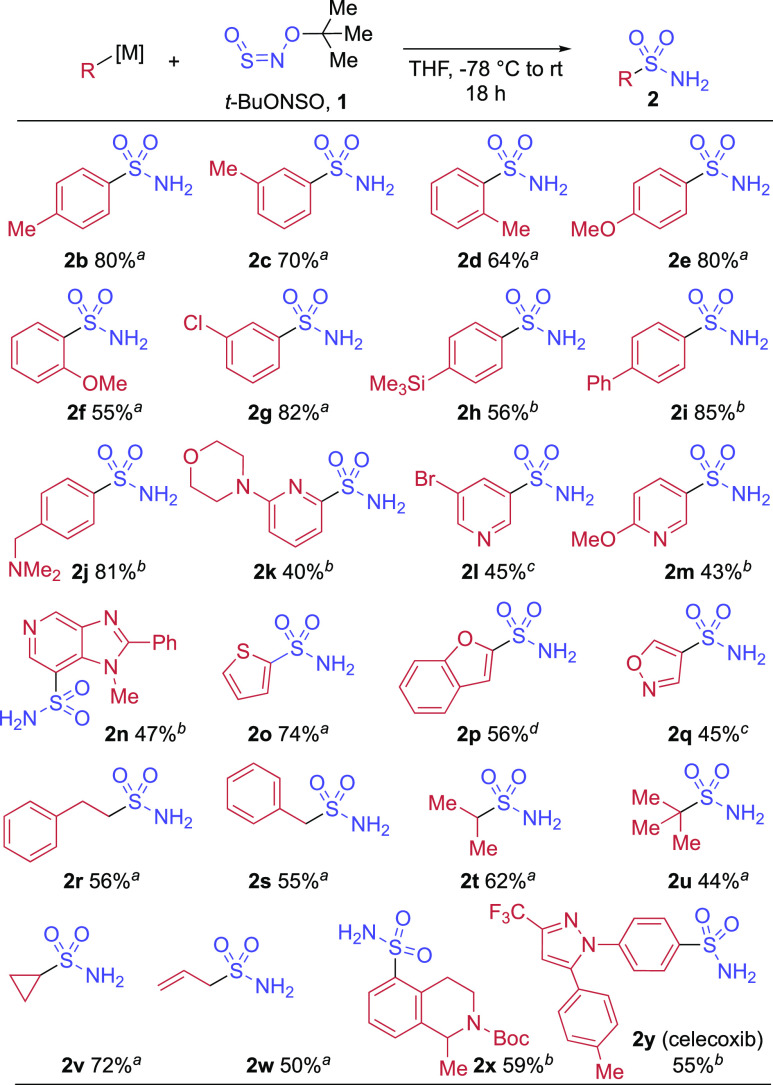
We were curious to see if this new reaction would prove general. Varying the aryl organometallic nucleophile confirmed that para-, meta-, and ortho-methyl substituents were all tolerated, with a minor drop in yield for the bulky o-tolylmagnesium bromide (2d) (Scheme 3). Using aryl nucleophiles with electron-donating and -withdrawing aryl groups delivered primary sulfonamides in high yields. A basic, and oxidatively sensitive tertiary amine could also be incorporated in excellent yield (2j). Turning to more medicinally relevant basic nitrogen heterocycles, we were pleased to find that 2- and 3-pyridyl sulfonamides, as well as a fused imidazopyridine, could all be prepared in synthetically useful yields (2k–2n). Five-membered heterocycles were also amenable to the reaction, with organometallic nucleophiles containing 2-thienyl, 2-benzofuranyl, and even the highly base-sensitive 4-isoxazolyl39 moiety all giving the desired primary sulfonamides (2o–2q). Alkyl organomagnesiums proved to be competent nucleophiles; steric factors did not affect the reaction significantly, with phenethyl, benzyl, isopropyl, and tert-butyl Grignard reagents all delivering product in moderate to good yields (2r–2u). Cyclopropylmagnesium bromide gave a higher yield of 72% (2v), and allylmagnesium bromide delivered the potentially sensitive sulfonamide 2w in 50% yield. The final two examples demonstrate that medicinally relevant structures can be readily prepared, with substituted tetrahydroisoquinoline 2x, a motif exploited by UCB in their dopamine receptor program,40 and celecoxib (2y), both obtained in workable yields.
Scheme 3. Scope of the Direct Primary Sulfonamide Synthesis.
Commercial solution of Grignard reagent used.
Organolithium reagent formed from aryl bromide and n-butyllithium.
Turbo Grignard reagent formed by coupling of aryl halide and i-PrMgCl.LiCl.
Organolithium reagent formed by deprotonation with n-butyllithium.
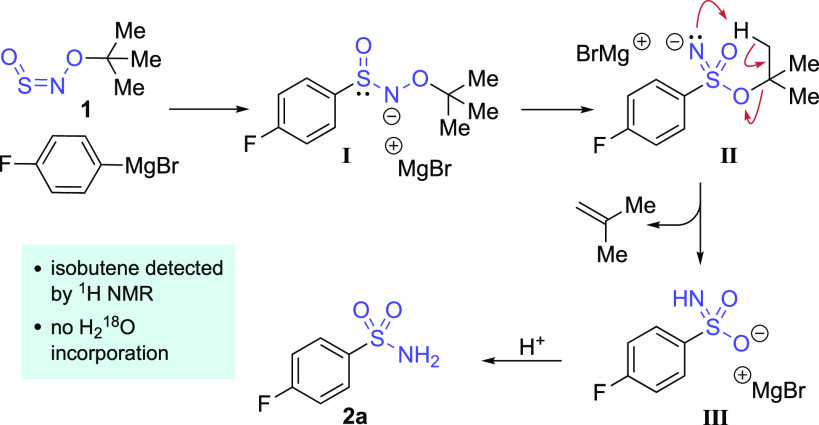
Preliminary mechanistic investigations have provided some insight into the mechanism of this unusual transformation, and our working model is shown in Scheme 4. Addition of the Grignard reagent to t-BuONSO 1 gives sulfinamide intermediate I, which then converts into sulfonimidate ester anion II, either via a sulfinyl nitrene intermediate36 or from a concerted N → S O-migration.41 An intramolecular proton transfer to the nitrogen atom proceeds to eliminate isobutene and give sulfonamide anion III, which is quenched upon workup to give the final sulfonamide product 2a. This proposal is supported by the lack of 18O incorporation when the reaction was quenched using 18O-labeled water at either −78 °C or room temperature, and by the observation of 1H NMR signals corresponding to isobutene in an aliquot of the crude reaction mixture (see Supporting Information for details). These preliminary experiments are consistent with both oxygen atoms of the sulfonamide originating from the t-BuONSO reagent.
Scheme 4. Proposed Mechanism for Sulfonamide Formation.
In summary, the development of the novel sulfinylamine reagent t-BuONSO 1 has led to a new synthesis of primary sulfonamides. Simply combining t-BuONSO with (hetero)aryl or alkyl organometallic nucleophiles such as Grignard reagents or oganolithiums gives rapid and convenient access to a broad range of medicinally relevant primary sulfonamides. We believe this method will find use as a straightforward way to install polarity and dramatically alter the physicochemical properties of molecules, starting from common alkyl and aryl halides.
Acknowledgments
This work was supported by the EPSRC Centre for Doctoral Training in Synthesis for Biology and Medicine (EP/L015838/1).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.0c03505.
Experimental procedures and supporting characterization data and spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Petkowski J. J.; Bains W.; Seager S. Natural Products Containing a Nitrogen-Sulfur Bond. J. Nat. Prod. 2018, 81, 423–446. 10.1021/acs.jnatprod.7b00921. [DOI] [PubMed] [Google Scholar]; b Mujumdar P.; Poulsen S. A. Natural Product Primary Sulfonamides and Primary Sulfamates. J. Nat. Prod. 2015, 78, 1470–1477. 10.1021/np501015m. [DOI] [PubMed] [Google Scholar]
- Bentley R. Different roads to discovery; Prontosil (hence sulfa drugs) and penicillin (hence beta-lactams). J. Ind. Microbiol. Biotechnol. 2009, 36, 775–786. 10.1007/s10295-009-0553-8. [DOI] [PubMed] [Google Scholar]
- Ansell B.; Clarke E. Acetazolamide in Treatment of Epilepsy. Br. Med. J. 1956, 1, 650–654. 10.1136/bmj.1.4968.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J. M.; Musini V. M.. First-line drugs for hypertension. Cochrane Db Syst. Rev. 2009. [DOI] [PubMed] [Google Scholar]
- Uddin M.; Rao P.; Knaus E. Design and synthesis of novel celecoxib analogues as selective cyclooxygenase-2 (COX-2) inhibitors: Replacement of the sulfonamide pharmacophore by a sulfonylazide bioisostere. Bioorg. Med. Chem. 2003, 11, 5273–5280. 10.1016/j.bmc.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Dahlen K.; Epstein D. L.; Grant W. M.; Hutchinson B. T.; Prien E. L.; Krall J. M. Repeated Dose-Response Study of Methazolamide in Glaucoma. Arch. Ophthalmol. 1978, 96, 2214–2218. 10.1001/archopht.1978.03910060516009. [DOI] [PubMed] [Google Scholar]
- Scott K. A.; Njardarson J. T. Top. Curr. Chem. (Z) 2018, 376, 5. 10.1007/s41061-018-0184-5. [DOI] [PubMed] [Google Scholar]
- Chen Y. T. Recent Functionalizations of Primary Sulfonamides. Synthesis 2016, 48, 2483–2522. 10.1055/s-0035-1562503. [DOI] [Google Scholar]
- Lang Y. Q.; Light P. E. Molecular Dissection of Sulfonylurea Pharmacogenomics in the ATP-Sensitive Potassium Channel Type 2 Diabetes Risk K23/A1369 Haplotype. Diabetes 2010, 59, A332–A332. [Google Scholar]
- Blair A. M.; Martin T. D. A Review of the Activity, Fate and Mode of Action of Sulfonylurea Herbicides. Pestic. Sci. 1988, 22, 195–219. 10.1002/ps.2780220303. [DOI] [Google Scholar]
- Dequirez G.; Pons V.; Dauban P. Nitrene Chemistry in Organic Synthesis: Still in Its Infancy?. Angew. Chem., Int. Ed. 2012, 51, 7384–7395. 10.1002/anie.201201945. [DOI] [PubMed] [Google Scholar]
- Fier P. S.; Maloney K. M. NHC-Catalyzed Deamination of Primary Sulfonamides: A Platform for Late-Stage Functionalization. J. Am. Chem. Soc. 2019, 141, 1441–1445. 10.1021/jacs.8b11800. [DOI] [PubMed] [Google Scholar]
- Reilly S. W.; Bennett F.; Fier P. S.; Ren S. M.; Strotman N. A. Late-Stage O-18 Labeling of Primary Sulfonamides via a Degradation-Reconstruction Pathway. Chem. - Eur. J. 2020, 26, 4251–4255. 10.1002/chem.202000484. [DOI] [PubMed] [Google Scholar]
- a Gómez-Palomino A.; Cornella J. Selective Late-Stage Sulfonyl Chloride Formation from Sulfonamides Enabled by Pyry-BF4. Angew. Chem., Int. Ed. 2019, 58, 18235–18239. 10.1002/anie.201910895. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Pérez-Palau M.; Cornella J. Synthesis of Sulfonyl Fluorides from Sulfonamides. Eur. J. Org. Chem. 2020, 2020, 2497–2500. 10.1002/ejoc.202000022. [DOI] [Google Scholar]
- Kerr W. J.; Reid M.; Tuttle T. Iridium-Catalyzed C-H Activation and Deuteration of Primary Sulfonamides: An Experimental and Computational Study. ACS Catal. 2015, 5, 402–410. 10.1021/cs5015755. [DOI] [Google Scholar]
- Sun X. Y.; Shan G.; Sun Y. H.; Rao Y. Regio- and Chemoselective C-H Chlorination/Bromination of Electron-Deficient Arenes by Weak Coordination and Study of Relative Directing-Group Abilities. Angew. Chem., Int. Ed. 2013, 52, 4440–4444. 10.1002/anie.201300176. [DOI] [PubMed] [Google Scholar]
- Zhu Q. L.; Graff D. E.; Knowles R. R. Intermolecular Anti-Markovnikov Hydroamination of Unactivated Alkenes with Sulfonamides Enabled by Proton-Coupled Electron Transfer. J. Am. Chem. Soc. 2018, 140, 741–747. 10.1021/jacs.7b11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire R. T.; Simon C. M.; Yadav A. A.; Ferguson M. J.; Stradiotto M. Nickel-Catalyzed Cross-Coupling of Sulfonamides With (Hetero)aryl Chlorides. Angew. Chem., Int. Ed. 2020, 59, 8952–8956. 10.1002/anie.202002392. [DOI] [PubMed] [Google Scholar]
- Kim T.; McCarver S. J.; Lee C.; MacMillan D. W. C. Sulfonamidation of Aryl and Heteroaryl Halides through Photosensitized Nickel Catalysis. Angew. Chem., Int. Ed. 2018, 57, 3488–3492. 10.1002/anie.201800699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X. H.; Xu H. Y.; Huang X. L.; Zhou J. S. Asymmetric Stepwise Reductive Amination of Sulfonamides, Sulfamates, and a Phosphinamide by Nickel Catalysis. Angew. Chem., Int. Ed. 2019, 58, 292–296. 10.1002/anie.201809930. [DOI] [PubMed] [Google Scholar]
- Diehl A. M.; Ouadoudi O.; Andreadou E.; Manolikakes G. Sulfonamides as Amine Component in the Petasis-Borono Mannich Reaction: A Concise Synthesis of alpha-Aryl- and alpha-Alkenylglycine Derivatives. Synthesis 2018, 50, 3936–3946. 10.1055/s-0037-1610440. [DOI] [Google Scholar]
- Bassin J. P.; Cremlyn R. J.; Swinbourne F. J. Chlorosulfonation of aromatic and heteroaromatic systems. Phosphorus, Sulfur Silicon Relat. Elem. 1991, 56, 245–275. 10.1080/10426509108038091. [DOI] [Google Scholar]
- Chen Y. D.; Murray P. R. D.; Davies A. T.; Willis M. C. Direct Copper-Catalyzed Three-Component Synthesis of Sulfonamides. J. Am. Chem. Soc. 2018, 140, 8781–8787. 10.1021/jacs.8b04532. [DOI] [PubMed] [Google Scholar]
- Woolven H.; Gonzalez-Rodriguez C.; Marco I.; Thompson A. L.; Willis M. C. DABCO-Bis(sulfur dioxide), DABSO, as a Convenient Source of Sulfur Dioxide for Organic Synthesis: Utility in Sulfonamide and Sulfamide Preparation. Org. Lett. 2011, 13, 4876–4878. 10.1021/ol201957n. [DOI] [PubMed] [Google Scholar]
- Laudadio G.; Barmpoutsis E.; Schotten C.; Struik L.; Govaerts S.; Browne D. L.; Noël T. Sulfonamide Synthesis through Electrochemical Oxidative Coupling of Amines and Thiols. J. Am. Chem. Soc. 2019, 141, 5664–5668. 10.1021/jacs.9b02266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham S. L.; Scholz T. H. The reaction of sulfinic acid salts with hydroxylamine-O-sulfonic aicd - A useful synthesis of primary sulfonamides. Synthesis 1986, 1986, 1031–1032. 10.1055/s-1986-31862. [DOI] [Google Scholar]
- Mendiola J.; Rincón J. A.; Mateos C.; Soriano J. F.; de Frutos O.; Niemeier J. K.; Davis E. M. Preparation, Use, and Safety of O-Mesitylenesulfonylhydroxylamine. Org. Process Res. Dev. 2009, 13, 263–267. 10.1021/op800264p. [DOI] [Google Scholar]
- a Buathongjan C.; Beukeaw D.; Yotphan S. Iodine-Catalyzed Oxidative Amination of Sodium Sulfinates: A Convenient Approach to the Synthesis of Sulfonamides under Mild Conditions. Eur. J. Org. Chem. 2015, 2015, 1575–1582. 10.1002/ejoc.201403531. [DOI] [Google Scholar]; b Yang K.; Ke M.; Lin Y.; Song Q. Sulfonamide formation from sodium sulfinates and amines or ammonia under metal-free conditions at ambient temperature. Green Chem. 2015, 17, 1395–1399. 10.1039/C4GC02236J. [DOI] [Google Scholar]
- Alvarez E. M.; Plutschack M. B.; Berger F.; Ritter T. Site-Selective C-H Functionalization-Sulfination Sequence to Access Aryl Sulfonamides. Org. Lett. 2020, 22, 4593–4596. 10.1021/acs.orglett.0c00982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo P. K. T.; Chen Y. D.; Willis M. C. Nickel(II)-Catalyzed Synthesis of Sulfinates from Aryl and Heteroaryl Boronic Acids and the Sulfur Dioxide Surrogate DABSO. ACS Catal. 2019, 9, 10668–10673. 10.1021/acscatal.9b04363. [DOI] [Google Scholar]
- a Feng J. B.; Wu X. F. A general iodine-mediated synthesis of primary sulfonamides from thiols and aqueous ammonia. Org. Biomol. Chem. 2016, 14, 6951–6954. 10.1039/C6OB01301E. [DOI] [PubMed] [Google Scholar]; b Hayashi E.; Yamaguchi Y.; Kita Y.; Kamata K.; Hara M. One-pot aerobic oxidative sulfonamidation of aromatic thiols with ammonia by a dual-functional β-MnO2 nanocatalyst. Chem. Commun. 2020, 56, 2095–2098. 10.1039/C9CC09411C. [DOI] [PubMed] [Google Scholar]
- Tota A.; St John-Campbell S.; Briggs E. L.; Estevez G. O.; Afonso M.; Degennaro L.; Luisi R.; Bull J. A. Highly Chemoselective NH- and O-Transfer to Thiols Using Hypervalent Iodine Reagents: Synthesis of Sulfonimidates and Sulfonamides. Org. Lett. 2018, 20, 2599–2602. 10.1021/acs.orglett.8b00788. [DOI] [PubMed] [Google Scholar]
- Kresze G.; Maschke A.; Bederke K.; Patzschke H.; Albrecht R.; Smalla H.; Trede A. Organische N-sulfinyl-Verbindungen. Angew. Chem. 1962, 74, 135–144. 10.1002/ange.19620740403. [DOI] [Google Scholar]
- Davies T. Q.; Hall A.; Willis M. C. One-Pot, Three-Component Sulfonimidamide Synthesis Exploiting the Sulfinylamine Reagent N-Sulfinyltritylamine. Angew. Chem., Int. Ed. 2017, 56, 14937–14941. 10.1002/anie.201708590. [DOI] [PubMed] [Google Scholar]
- Zhang Z. X.; Davies T. Q.; Willis M. C. Modular Sulfondiimine Synthesis Using a Stable Sulfinylamine Reagent. J. Am. Chem. Soc. 2019, 141, 13022–13027. 10.1021/jacs.9b06831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies T. Q.; Tilby M. J.; Ren J.; Parker N. A.; Skolc D.; Hall A.; Duarte F.; Willis M. C. Harnessing Sulfinyl Nitrenes: A Unified One-Pot Synthesis of Sulfoximines and Sulfonimidamides. J. Am. Chem. Soc. 2020, 142, 15445–15453. 10.1021/jacs.0c06986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricich T. J.; Hoffman V. L. Chemistry of Benzenesulfinyl Azides - Reactions with sulfoxides. J. Am. Chem. Soc. 1974, 96, 7770–7781. 10.1021/ja00832a026. [DOI] [Google Scholar]
- t-BuONSO is available from Cortex Organics (cortexorganics.com).
- Morita T.; Fuse S.; Nakamura H. Generation of an 4-Isoxazolyl Anion Species: Facile Access to Multifunctionalized Isoxazoles. Angew. Chem., Int. Ed. 2016, 55, 13580–13584. 10.1002/anie.201608039. [DOI] [PubMed] [Google Scholar]
- a Valade A.; Jnoff E.; Ates A.; Burssens P.; Skolc D.. Tetrahydroisoquinoline Derivatives, WO 2016/055479 A1.; b Hall A.; Provins L.; Valade A. Novel Strategies To Activate the Dopamine D1 Receptor: Recent Advances in Orthosteric Agonism and Positive Allosteric Modulation. J. Med. Chem. 2019, 62, 128–140. 10.1021/acs.jmedchem.8b01767. [DOI] [PubMed] [Google Scholar]
- Leca D.; Fensterbank L.; Lacôte E.; Malacria M. A New Practical One-Pot Access to Sulfonimidates. Org. Lett. 2002, 4, 4093–4095. 10.1021/ol026837b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.