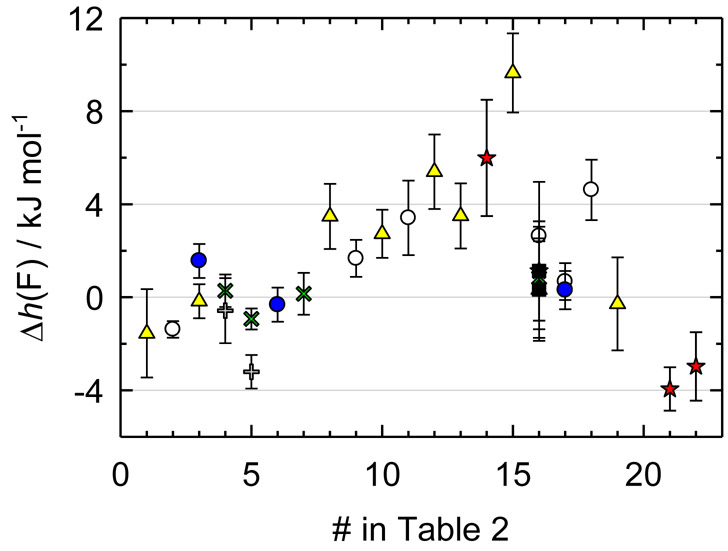
Figure 1.
Relative effective enthalpy, Δh(F) / (kJ·mol−1) = h(F) / (kJ·mol−1) + 261711.5, of a fluorine atom for the medium-size organic molecules as a function of the sequential number of a compound in Table 2: blue circles, Moscow lab; yellow triangles, Porto lab (1997-2014); green crosses, Teddington lab; gray pluses, Windsor lab; red stars, Freiburg lab; empty circles, Bartesville; black squares, Gaithersburg. The value for 5-fluoro-2-methylbenzoxazole (#20) is not shown due to a large deviation (−8.6 kJ·mol−1). For 5-fluorouracil (#1) and (trifluoromethyl)benzene (#17) only the most recent values for laboratories are shown.