FIG. 1.
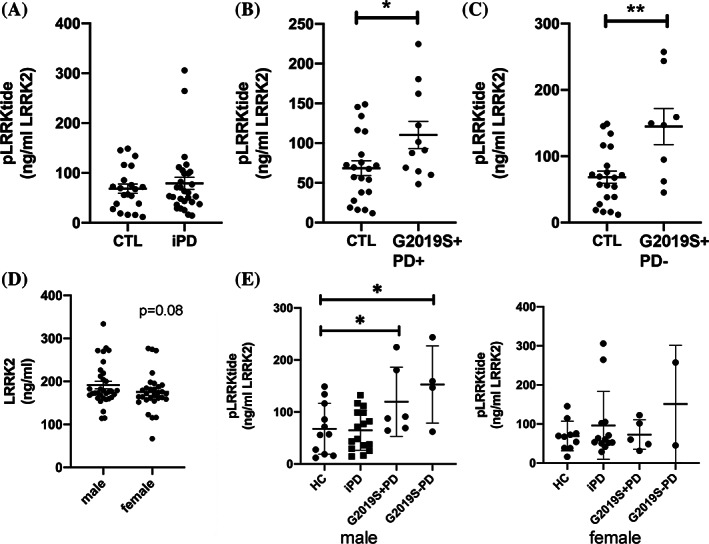
LRRK2 in vitro kinase activity in PBMC extracts is increased in carriers of the G2019S mutation. We measured in vitro kinase activity using a model peptide substrate in an in‐well kinase reaction. Phosphorylation of the peptide was normalized to the total amount of LRRK2 in each well. We could find no significant change in the intrinsic kinase activity of LRRK2 in PBMCs of iPD patients (A); however, phosphorylation was significantly increased in affected (B) and healthy (C) carriers of the G2019S‐LRRK2 mutation (middle and right panels). Mann‐Whitney U test, *P < 0.05; **P < 0.01. (D) LRRK2 expression and in vitro activity were compared in PBMCs from males and females. In samples from healthy control and iPD patients, we found a trend for higher levels of expression in PBMCs from males compared to females; Mann‐Whitney U, P = 0.08 (Ctl). Interestingly, the increased in vitro kinase activity detected in PBMCs from carriers of the G2019S‐LRRK2 mutation, when analyzed separately by sex, was only evident in PBMCs from male subjects (E). The activity of LRRK2 from PBMCs of female G2019S carriers is not different from healthy controls. *P < 0.05. CTL, control; HC, healthy controls; pLRRKtide, phosphorylation of the LRRKtide substrate.
