Abstract
Background
The two previous versions of the Scandinavian donations and transfusions (SCANDAT) databases, encompassing data on blood donors, blood components, transfusions, and transfused patients linked to national health registers in Sweden and Denmark up until 2012, have been used to study donor health, disease transmission, the role of donor characteristics, and more.
Study Design and Methods
Here we describe the creation of the Swedish portion of the third iteration of SCANDAT – SCANDAT3‐S – with follow‐up from 1968 to the end of 2017, resulting in up to 50 years of uninterrupted follow‐up for donors and recipients. The database now also includes non‐transfused non‐donors with a blood typing result, increased temporal resolution for transfusions, and linkages to laboratory and drug prescription data.
Results
After data cleaning, the database contained 23 579 863 donation records, 21 383 317 transfusion records, and 8 071 066 unique persons with valid identification. In total, the database offers 28 638 436 person‐years of follow‐up for donors, 13 582 350 person‐years of follow‐up for transfusion recipients, and 65 613 639 person‐years of follow‐up for non‐recipient non‐donors, with possibility for future extension. Additionally, the database includes 167 820 412 dispense records for prescribed drugs and 316,338,442 laboratory test results. Since the latest update in 2012, >99.9% of all donations were traceable to a donor with valid identification, and >97% of all transfusions to a recipient with valid identification.
Conclusion
With extended follow‐up and more clinical detail, the Swedish portion of the third and latest iteration of the SCANDAT database should allow for more comprehensive analysis of donation and transfusion‐related research questions.
1. INTRODUCTION
The Swedish‐Danish Scandinavian Donation and Transfusions (SCANDAT) databases have been a series of “vein to vein” databases with detailed and linked data on donors, donations, blood components, transfusions, and transfusion recipients. The original version contained data from 1968 to 2002, with essentially full binational coverage from 1996 (Sweden) and 1998 (Denmark) onwards. The database has since been updated once with data up to 2012 in its second iteration, SCANDAT2. Leveraging the detail and coverage of Scandinavian population and health registers, the SCANDAT databases have offered virtually complete life‐time follow‐up for both donors and recipients, with particular strengths in the diagnoses of severe diseases through national inpatient and cancer registers.
The SCANDAT databases have been used to conduct large‐scale transfusion epidemiological studies that have shown: no evidence of transmission of malignancies, neurodegenerative disorders, celiac disease, and unknown hepatitis viruses 1 , 2 , 3 , 4 ; no evidence of an effect of storage time, sex‐concordant blood units, donor age, donor sex, and donor parity on the mortality of recipients of RBC transfusions 5 , 6 , 7 , 8 , 9 , 10 , 11 ; increased short‐term mortality in recipients of female plasma 12 ; no increased risk of polycythemia in whole blood donors, or risk of fractures in apheresis donors 9 , 13 ; correlation between ABO group and cancer, thromboembolic and arterial disease, gastric cancer and peptic ulcers, and the lack of association with dementia. 13 , 14 , 15 , 16 , 17 Furthermore, we have published population‐based descriptive studies on blood use, as well as economic evaluations of blood‐screening, among others. 18 , 19
Limitations to previous iterations of SCANDAT include: inability to capture less‐severe conditions that are typically not captured by patient registers lacking primary care coverage; having only the transfusion date and not the time and order of transfused blood products to capture the iterative decision‐making process and potential time‐dependent confounding during a single day; lack of routine blood chemistry data; as well as only containing transfused patients without the possibility to characterize non‐transfused patients in greater detail.
To allow for more comprehensive transfusion epidemiological research and in an effort to remedy some of said limitations, we redesigned and updated the SCANDAT database to improve its utility and extend follow‐up even further. To fully leverage local health care data resources, the Swedish and Danish parts of SCANDAT3 will be stored as structurally similar but separate databases. This paper covers the Swedish part of SCANDAT3, hereinafter referred to as SCANDAT3‐S.
2. MATERIALS AND METHODS
2.1. Data sources
Computerized records of blood donation, transfusion, and transfusion medicine related laboratory results were extracted from local laboratory information management systems (“local transfusion databases”). In Sweden, 20 regions used various versions of Prosang (CSAM Prosang AB, Stockholm, Sweden) and one region used Flexlab/DoReMi (Tieto Sweden AB, Stockholm, Sweden).
As before, all inhabitants of Sweden are assigned national registration numbers (NRN), which both uniquely identify persons and allow linkages to all other health and population registers.
Laboratory results for inpatients and hospital‐associated outpatient clinics in Stockholm, Sweden were extracted through the electronic health records (EHR) from all emergency hospitals. In Stockholm, one hospital used Cambio COSMIC (Cambio Healthcare Systems AB), and the remaining three hospitals used TakeCare (Compugroup Medical Sweden AB, Uppsala, Sweden). For the three latter hospitals, laboratory results from bedside tests that have been inputted manually to the EHR were separately extracted, typically capillary blood tests or blood gas analyses from earlier years before analysis equipment were directly linked to local EHRs.
Additional laboratory results from tests ordered in primary care were extracted directly from the laboratory information management systems of the largest laboratory in Stockholm that used Flexlab (Tieto Sweden AB, Stockholm, Sweden). Data extraction from the remaining laboratories in Stockholm, as well as the other major cities in Sweden is ongoing.
2.2. Data collection
The data collection from local transfusion databases took place between 2017‐2019 and the process was largely similar to previous versions of SCANDAT (Figure A1). Ethical approval from the regional ethical committee were acquired prior to the data collection. After acquiring approval from register holders for the data extraction, typically one per municipality, extraction scripts were written and run remotely by transfusion laboratory information management system suppliers per our specifications. Register holders formally requested the extraction from the laboratory information management system supplier and also handled the file transfer. Encrypted data were transmitted to Karolinska Institutet through a secure file transfer system, RMFT (RepliWeb Managed File Transfer, Attunity Inc, Burlington, MA).
For the data collection of laboratory test scripts were written by the IT departments of each hospital for extraction from EHR, and by laboratory IT for extraction from blood chemistry laboratory information management system, after receiving approval from the regional ethics committee and register holders. The extraction scripts were designed to only extract laboratory test results from individuals in SCANDAT3‐S and included only the prespecified analyses of interest. Laboratory data were transmitted directly to Statistics Sweden using encrypted FTP‐service.
2.3. Data processing, record linkages, and database design
Database processing of data from local transfusion databases was largely similar to previous versions of SCANDAT. In short, obviously erroneous records and duplicates were removed, and different coding systems for eg, donation and component types, were harmonized as needed. After data cleaning and harmonization at Karolinska Institutet, the database was sent to Statistics Sweden, the government agency for population statistics, that subsequently pseudonymized the dataset by replacing NRNs by random unique personal identification numbers (PIDs). Similarly, laboratory data that was sent directly to Statistics Sweden was pseudonymized using the same key. In parallel, data was also linked with national health registers at the Swedish Board of Health and Welfare using NRNs, with health outcomes until the end of 2017 and national population registers by Statistics Sweden with vital status and demographic data until the end of 2018 (Table A1), before being transmitted back to Karolinska Institutet. The final key‐file is stored at the Swedish Board of Health and Welfare to allow for database updates in the future.
The final database design is similar to SCANDAT2 (Figure A2). Details of the nationwide population and health data registers have been described previously. 20 , 21
2.4. Statistical analyses
The total number of donors, transfusion‐recipients and non‐transfusion recipients non‐donors were counted separately based on status at the end of follow‐up, ie, donors who have also received transfusions are counted in both, whereas those that had neither donated or received transfusions at the end of follow‐up were counted in the last group Follow up time was calculated starting from first donation (donors), first transfusion (recipients), or first blood typing (non‐recipients and non‐donors), until first emigration, death, or 31 December 2018.
The total number of all recorded types of transfusions and successful donations was counted annually, the latter including whole blood, plasmapheresis, plateletpheresis and red‐cell apheresis donations. Transfusions or donations from individuals whom had erroneously formatted or recorded NRNs, missing linkages to the population registers of Statistic Sweden, reused NRNs, or conflicting sex and/or blood types were flagged as non‐traceable. Three types of traceability were calculated: the proportion of all donations with known donor, the proportion of all transfusions with known donor, and the proportion of all transfusions with known recipient.
The total number of laboratory results extracted from EHRs of all emergency hospitals and the largest laboratory in Stockholm, Sweden were counted based on unique combinations of PID, calendar time, result, and analysis type.
The conduct of this study was approved by the regional ethics review board at Karolinska Institutet in Stockholm, Sweden (2018/167‐31, 2019‐02636). All data management and statistical analyses was performed using SAS statistical analysis software, version 9.4 (SAS Institute, Cary, NC).
3. RESULTS
Data from local transfusion databases were extracted from all 21 regional databases in Sweden. In total, 23 579 863 donation records and 21 383 317 transfusion records remained after data cleaning. After removing erroneous NRNs, 8 071 066 persons remained, of whom 1 475 262 ever donated blood, 1 873 447 ever received a blood transfusion, and the remaining 4 821 591 neither donated blood nor received a transfusion as of the end of follow‐up.
The database provides 28 638 436 person‐years of follow‐up for donors, and with over 40 years of follow up for 113 802 individual donors; 13 582 350 person‐years of follow‐up for transfusion recipients and over 40‐year of follow up for 25 271 individual transfusion‐recipients; as well as 65 613 639 person‐years of follow‐up for non‐transfusion‐recipients and non‐donors (Table 1).
TABLE 1.
Number of individuals and follow‐up for individuals in SCANDAT3‐S by exposure status at the end of follow‐up. Follow up was calculated from first donation for individuals that have donated at least one unit (donors), from first transfusion for individuals that have received at least one transfusion (recipients), and from first blood typing for individuals that had neither received a transfusion or donated blood at end of follow‐up (non‐recipient non‐donors)
| Donors | Transfusion recipients | Non‐donors/transfusion recipients as of end of follow‐up | |
|---|---|---|---|
| Sex, N (%) | 1 475 262 | 1 873 447 | 4 821 591 |
| Male | 755 994 (51%) | 825 401 (44%) | 1 976 263 (41%) |
| Female | 719 268 (49%) | 1 048 046 (56%) | 2 845 328 (59%) |
| Age at entry, years (SD) | 30.8 (11.4) | 64.6 (21.4) | 39.1 (24.1) |
| Follow‐up, N | |||
| 0‐4 y | 232 581 | 996 970 | 1 267 788 |
| 5‐9 y | 197 205 | 333 979 | 808 926 |
| 10‐14 y | 163 238 | 209 271 | 644 100 |
| 15‐19 y | 180 343 | 132 956 | 594 860 |
| 20‐29 y | 353 439 | 123 263 | 1 118 628 |
| 30‐39 y | 234 654 | 51 737 | 372 011 |
| 40+ y | 113 802 | 25 271 | 15 278 |
| Total follow‐up | 28 638 436 | 13 582 350 | 65 613 639 |
Abbreviations: SD, standard deviation; y, years.
For donors, the mean age at first recorded donation was 30.8 years (Standard Deviation [SD], 11.4), the mean age at first recorded transfusion for recipients was 64.6 years (SD, 21.4), and the mean age of first recorded blood typing for non‐recipient non‐donors as of end of follow‐up was 39.1 years (SD, 24.1).
The annual number of donations and transfusions recorded in SCANDAT3‐S is presented in Figure 1. Transfusion and donation data were mostly available until November 2018 or later, although 7 out of 21 blood centers provided data until between August and October, 2018. The sudden drop in the number of donations in 2004 is due to the cessation of plasma collection for the purpose of fractionation in select regions. Vital status was available to 31 December 2018 for all individuals.
FIGURE 1.
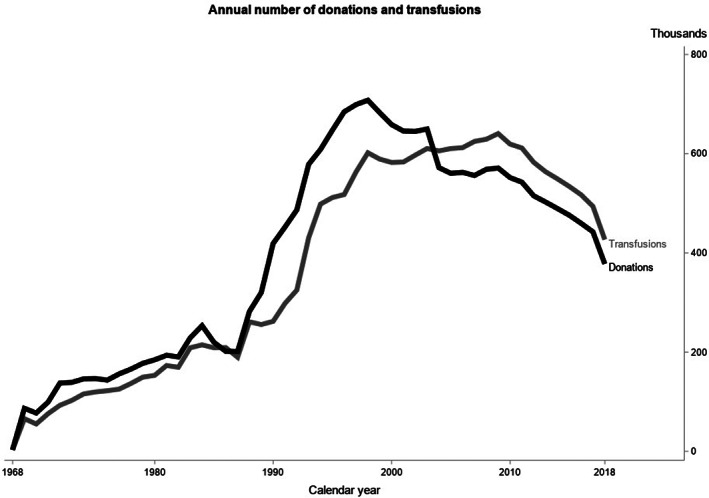
Number of donations and transfusions recorded in the SCANDAT3‐S database
Traceability of donors and recipients in SCANDAT3S is presented in Figure 2. Historical variations in traceability prior to 2012 has previously been described, and was mostly due to damage or loss of magnetic data tapes in the mid 1980's. After 2012, the traceability of donors to donations continues to be high; <0.1% are untraceable likely due to a very small number of donors having a reused NRN, or for rarer reasons like conflicting blood typing due to weak or partial D phenotypes. The small proportion of transfusions without a traceable donor (<2%) is largely due transfusions from solvent detergent plasma pooled from up to over 1000 donors, where the donor is not recorded in the transfusion databases, and imported blood products. Finally, the proportion of transfusions without a traceable recipient (<3%) is mostly attributed to blood products that were ordered in an emergency setting to yet identified patients. In general, traceability for both donors and recipients for donations and transfusions is very high, particularly from 1996 onwards.
FIGURE 2.
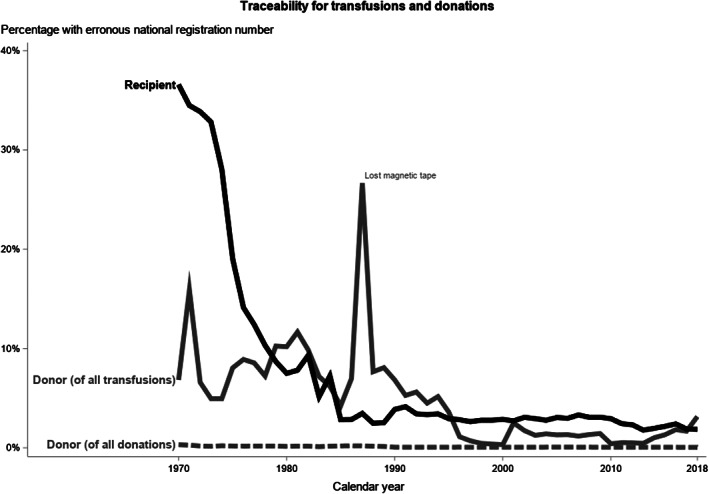
Number of donations and transfusions traceable to a donor or recipient with valid identification in the SCANDAT3‐S database
The availability of data from linkages to other registers is shown in Figure 3. Nationwide data from the Medical Birth Register was available starting in 1973, inpatient hospital discharge data from the National Patient Register was available nationwide starting from 1986, and outpatient starting from 2001. Blood typing from non‐recipient non‐donors was available from 1978 and had coverage for 20 out of 21 regions throughout most of the 1990s, with full national coverage starting in 2000.
FIGURE 3.
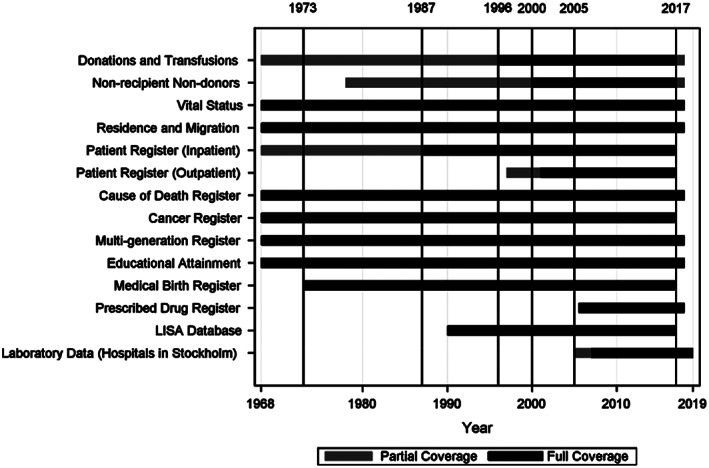
Data availability for selected linked registers and datasets over time in SCANDAT3‐S
Laboratory records in SCANDAT3‐S are shown in Table 2. In total, 316 338 442 blood chemistry test results, including 5 268 218 manually entered test results, from all emergency hospitals in Stockholm were extracted for 1 623 726 individuals in the cohort with coverage from 2005 to the beginning of 2019. Additionally, the database includes 167 820 412 dispense records for prescribed drugs from July 2005 to the end of 2018, with full national coverage.
TABLE 2.
Blood chemistry test results currently available in SCANDAT3‐S
| Group | Tests | Records, N |
|---|---|---|
| Complete blood count | Hemoglobin concentration, MCV, MCH, MCHC, platelets, leukocytes, neutrophils, reticulocytes, leukocyte differential | 135 610 890 |
| Iron | Ferritin, transferrin, transferrin saturation, ferritin, iron | 4 136 820 |
| Inflammation | C‐Reactive Protein, Sedimentation Rate | 16 600 977 |
| Hemolysis | Haptoglobin, Lactate Dehydrogenase, Bilirubin | 7 798 425 |
| Cardiac | Cardiac Troponin, NT‐proBNP | 2 270 123 |
| Kidney and electrolytes | Potassium, Sodium, Calcium, Chlorine, Creatinine, Urea | 64 425 362 |
| Liver | ALT, AST, ALP, GGT, Albumin | 30 871 221 |
| Coagulation | Fibrinogen, INR, aPTT | 8 880 427 |
| Metabolic | HbA1c, Cholesterol, LDL, HDL, Triglycerides, Glucose | 29 481 199 |
| Others | ABG, VBG, Lactate, etc. | 16 262 998 |
4. DISCUSSION
SCANDAT3‐S is the Swedish part of the latest iteration of the SCANDAT databases, which also incorporates drug prescription data, routine laboratory results, time and order of transfusions, as well as data on persons who underwent blood typing but did not donate blood nor receive a transfusion. The new database offers up to a maximum of 50 years of uninterrupted follow‐up of both donors and transfused patients, with data complete until 2018. Data quality continues to be high, with less than 2% of transfusion being untraceable to identifiable donor(s) and with more than 97% of transfusions being recorded as having been administered to identifiable patients. In recent years, almost 100% of donations are traceable to its donor.
Data on prescribed drugs in SCANDAT3‐S will allow for more comprehensive studies in several areas. Antimicrobial prescription data allow for more sensitive analyses on potential immunosuppression, with the possibility to capture less severe infections typically not captured by inpatient or secondary care outpatient registers. Information on anticoagulation and antiplatelet drug use will be informative in studying transfusions in patient groups with or at risk of bleeding. Furthermore, data on the use of iron supplementation, erythropoietin, and immunostimulants will be particularly important in select patient groups, such as those with anemia and hematological malignancies.
The inclusion of routine blood chemistry laboratory results opens up the possibility for answering a new range of research questions. Complete blood counts allow for the study of determinants of transfusion efficacy, as well as studies on transfusion thresholds. Conventional coagulation test results allow for more detailed studies, perhaps most relevant in patients with bleeding, and trauma‐induced coagulopathy. Apart from being used for analyses directly, we also expect the laboratory results to helpful for constructing more robust disease definitions, ie, being used to confirm diagnoses extracted from the patient registers, such as for myocardial infarction, diabetes, or organ failure.
Increased temporal resolution allows the order of transfusions to be ascertained, allowing for more complex modeling that consider time‐dependent effects and time‐dependent confounding, for example using marginal structural models. This may be particularly relevant in studying the effect of donor characteristics in transfused patients who receive more than one unit per day. Timing of donations may also allow for studies of donor behavior which may aid with donor recruitment and retention.
Lastly, the inclusion of individuals who have undergone blood typing but have not received a transfusion or donated blood will allow us to also perform detailed analyses of patients who were deemed at risk of being transfused, but who did not receive a transfusion, such as obstetric patients or patients undergoing elective surgery. This may allow more relevant comparators for transfusion recipients and studies on blood transfusion determinants.
Several other transfusion databases exists, such as the Dutch Transfusion Data warehouse, the successor to the Profile of Transfusion Recipients (PROTON databases), 22 data from Kaiser Permanente Northern California, 23 the Recipient Epidemiology and Donor Evaluation Study (REDS) programs, 24 as well as ad‐hoc datasets created by linking blood collection agency and clinical data. 25 As far as we know, the SCANDAT databases are still unique in both its population‐level coverage, as well as the ability to link the donation‐transfusion data with other population and health registers that offer detailed life‐time follow‐up for donors, recipients, and now also all persons with a blood typing result.
Still, there are several limitations that should be considered. In the study of transfusions, perhaps the largest limitation is the lack of recorded transfusion indication. Instead, indication for transfusions have to be deduced based on diagnosis and surgery codes, which leaves room for misclassification and residual confounding by indication. In addition, dues to the sheer amount of data gathered over a long time, analytical approaches are often very challenging and need to consider data entry errors and misclassification, inconsistencies, and changes over time. Furthermore, findings using the SCANDAT databases may be limited in generalizability to countries with drastically different donation and/or transfusion practices.
In general, transfusion reactions that do not lead to death or other diagnosable outcomes are not well defined and will be difficult to study with existing data. While, the inclusion of laboratory data can be used to circumvent some of these challenges, it is still hard to detect milder effects of transfusions. As such, we have completed linkage with a number of quality registers, eg, the Swedish intensive care register, to enable more nuanced analyses. 26 Additionally, we are unable to trace solvent detergent plasma units to their donors, which will become an increasingly problematic issue as solvent detergent plasma becomes more common in clinical use in Sweden. 27 Finally, in the study of donor health, it is still analytically challenging to fully account for the “healthy donor effect” due to possible reverse causality between potentially ill‐effects and willingness to donate, as well as strong self‐selection mechanisms. 28
In conclusion, SCANDAT3‐S is the Swedish part of the latest and most comprehensive iteration of the SCANDAT databases, with up to 50‐years of uninterrupted life‐time follow‐up for donors, recipients, and patients at risk for transfusions.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
We are indebted to all the blood banks in Sweden for both collecting and contributing data to this study. We would like to thank Södersjukhuset, Danderydʼs Hospital, Karolinska University Hospital, Capio S:t Göranʼs Hospital, and Karolinska University Laboratory for providing laboratory data. We would also like to thank Stella Karsten (Department of Transfusion Medicine and Clinical Immunology, Karolinska University Hospital) for assistance with ISBT‐codes. We are especially thankful to CSAM Prosang for their enduring support of the SCANDAT databases and Josefin Journath (Statistics Sweden).
Appendix A.
FIGURE A1.
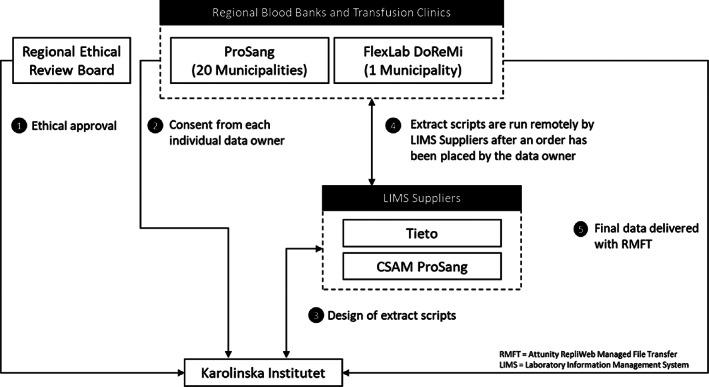
Data collection process
TABLE A1.
Description of data from linked population and health registers
| Description of contents | |
|---|---|
| Population registers | |
| Multi‐generation register | First‐order relatives that are also in the cohort |
| Total population register | Domestic migrations, emigration, immigration, vital status, date of death, birthyear and month, sex |
| LISA database | Education, profession, income |
| Others | |
| Health registers | |
| Inpatient and outpatient registers | Visits, inpatient episodes, diagnosis, interventions |
| Drug prescription register | Drug type, package size, date of pick‐up |
| Cause of death register | Cause of death, obduction, death date |
| Medical birth register | Detailed information on delivery of offspring and neonatal health status |
| Cancer register | Diagnosis, histopathology, TNM |
FIGURE A2.
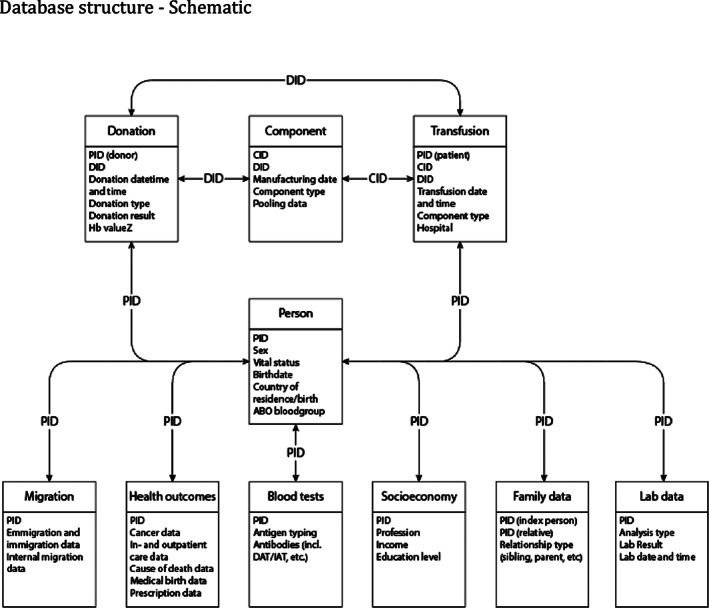
Database structure – schematic
Zhao J, Rostgaard K, Hjalgrim H, Edgren G. The Swedish Scandinavian donations and transfusions database (SCANDAT3‐S) – 50 years of donor and recipient follow‐up. Transfusion. 2020;60:3019–3027. 10.1111/trf.16027
Funding information Karolinska Institutet; Region Stockholm; Vetenskapsrådet, Grant/Award Number: 2017‐01954; The creation of the SCANDAT database and the conduct of this study was made possible by a grant to Dr Edgren from Swedish Research Council, Grant/Award Number: 2017‐01954; Dr Edgren is supported by Region Stockholm (clinical research appointment). Dr Zhao is supported by the Clinical Scientist Training Program and the Research Internship Program, both at Karolinska Institutet.
REFERENCES
- 1. Hjalgrim H, Rostgaard K, Vasan SK, et al. No evidence of transmission of chronic lymphocytic leukemia through blood transfusion. Blood. 2015;126:2059–2061. [DOI] [PubMed] [Google Scholar]
- 2. Edgren G, Hjalgrim H, Reilly M, et al. Risk of cancer after blood transfusion from donors with subclinical cancer: a retrospective cohort study. Lancet. 2007;369:1724–1730. [DOI] [PubMed] [Google Scholar]
- 3. Edgren G, Hjalgrim H, Rostgaard K, et al. Searching for unknown transfusion‐transmitted hepatitis viruses: a binational cohort study of 1.5 million transfused patients. J Intern Med. 2018;284:92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Edgren G, Hjalgrim H, Rostgaard K, et al. Transmission of neurodegenerative disorders through blood transfusion: a cohort study. Ann Intern Med. 2016;165:316–324. [DOI] [PubMed] [Google Scholar]
- 5. Holzmann MJ, Sartipy U, Olsson ML, Dickman P, Edgren G. Sex‐discordant blood transfusions and survival after cardiac surgery: a nationwide cohort study. Circulation. 2016;134:1692–1694. [DOI] [PubMed] [Google Scholar]
- 6. Edgren G, Murphy EL, Brambilla DJ, et al. Association of blood donor sex and prior pregnancy with mortality among red blood cell transfusion recipients. JAMA. 2019;321:2183–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Edgren G, Rostgaard K, Vasan SK, et al. The new Scandinavian donations and transfusions database (SCANDAT2): a blood safety resource with added versatility. Transfusion. 2015;55:1600–1606. [DOI] [PubMed] [Google Scholar]
- 8. Edgren G, Ullum H, Rostgaard K, et al. Association of donor age and sex with survival of patients receiving transfusions. JAMA Intern Med. 2017;177:854–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grau K, Vasan SK, Rostgaard K, et al. No association between frequent apheresis donation and risk of fractures: a retrospective cohort analysis from Sweden. Transfusion. 2017;57:390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Halmin M, Rostgaard K, Lee BK, et al. Length of storage of red blood cells and patient survival after blood transfusion: a binational cohort study. Ann Intern Med. 2017;166:248–256. [DOI] [PubMed] [Google Scholar]
- 11. Sartipy U, Holzmann MJ, Hjalgrim H, Edgren G. Red blood cell concentrate storage and survival after cardiac surgery. JAMA. 2015;314:1641–1643. [DOI] [PubMed] [Google Scholar]
- 12. Tynell E, Andersson TM, Norda R, et al. Should plasma from female donors be avoided? A population‐based cohort study of plasma recipients in Sweden from 1990 through 2002. Transfusion. 2010;50:1249–1256. [DOI] [PubMed] [Google Scholar]
- 13. Edgren G, Nyren O, Hultcrantz M, et al. Blood donation and risk of polycythemia vera. Transfusion. 2016;56:1622–1627. [DOI] [PubMed] [Google Scholar]
- 14. Edgren G, Hjalgrim H, Rostgaard K, et al. Risk of gastric cancer and peptic ulcers in relation to ABO blood type: a cohort study. Am J Epidemiol. 2010;172:1280–1285. [DOI] [PubMed] [Google Scholar]
- 15. Vasan SK, Hwang J, Rostgaard K, et al. ABO blood group and risk of cancer: a register‐based cohort study of 1.6 million blood donors. Cancer Epidemiol. 2016;44:40–43. [DOI] [PubMed] [Google Scholar]
- 16. Vasan SK, Rostgaard K, Majeed A, et al. ABO blood group and risk of thromboembolic and arterial disease: a study of 1.5 million blood donors. Circulation. 2016;133:1449–1457. discussion 57. [DOI] [PubMed] [Google Scholar]
- 17. Vasan SK, Rostgaard K, Ullum H, Melbye M, Hjalgrim H, Edgren G. ABO blood group and dementia Risk—a Scandinavian record‐linkage study. PLoS One. 2015;10:e0129115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kamper‐Jorgensen M, Hjalgrim H, Edgren G, et al. Expensive blood safety initiatives may offer less benefit than we think. Transfusion. 2010;50:240–242. [DOI] [PubMed] [Google Scholar]
- 19. Zhao J, Ryden J, Wikman A, et al. Blood use in hematologic malignancies: a nationwide overview in Sweden between 2000 and 2010. Transfusion. 2018;58:390–401. [DOI] [PubMed] [Google Scholar]
- 20. Ludvigsson JF, Otterblad‐Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thygesen LC, Ersboll AK. Danish population‐based registers for public health and health‐related welfare research: introduction to the supplement. Scand J Public Health. 2011;39:8–10. [DOI] [PubMed] [Google Scholar]
- 22. van Hoeven LR, Hooftman BH, Janssen MP, et al. Protocol for a national blood transfusion data warehouse from donor to recipient. BMJ Open. 2016;6:e010962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karafin MS, Bruhn R, Westlake M, et al. Demographic and epidemiologic characterization of transfusion recipients from four US regions: evidence from the REDS‐III recipient database. Transfusion. 2017;57:2903–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roubinian NH, Murphy EL, Swain BE, Gardner MN, Liu V, Escobar GJ. Predicting red blood cell transfusion in hospitalized patients: role of hemoglobin level, comorbidities, and illness severity. BMC Health Serv Res. 2014;14:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chasse M, Tinmouth A, English SW, et al. Association of blood donor age and sex with recipient survival after red blood cell transfusion. JAMA Intern Med. 2016;176:1307–1314. [DOI] [PubMed] [Google Scholar]
- 26. Sjöberg F, Walther S. Intensive care registries and the evolution of the concept of 'quality of care' ‐ reflections from the 10‐year anniversary symposium of the Swedish Intensive Care Registry. Acta Anaesthesiol Scand. 2012;56:1073–1077. [DOI] [PubMed] [Google Scholar]
- 27. Marietta M, Franchini M, Bindi ML, Picardi F, Ruggeri M, De Silvestro G. Is solvent/detergent plasma better than standard fresh‐frozen plasma? A systematic review and an expert consensus document. Blood Transfus. 2016;14:277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Edgren G, Tran TN, Hjalgrim H, et al. Improving health profile of blood donors as a consequence of transfusion safety efforts. Transfusion. 2007;47:2017–2024. [DOI] [PubMed] [Google Scholar]


