Abstract
Drug‐induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic symptoms (DRESS) is a severe adverse drug reaction characteristically associated with sequential reactivation of herpesviruses, such as human herpesvirus 6 (HHV‐6), Epstein‐Barr virus (EBV), and cytomegalovirus (CMV). Since systemic corticosteroids are thought to result in viral reactivation due to their immunosuppressive effects, we clarified the influence of systemic corticosteroid therapy on viral reactivation in DIHS/DRESS. Viral DNA in peripheral whole blood and serum sIL‐2R level were measured during the disease course in twenty DIHS/DRESS patients. Six of seven patients treated without corticosteroids experienced HHV‐6 viremia associated with elevated serum sIL‐2R levels. In contrast, high‐dose corticosteroids started within 1 week after onset tended to inhibit the occurrence of HHV‐6 reactivation with remarkable suppression of serum sIL‐2R level. Low‐dose corticosteroids or late‐start high‐dose corticosteroids did not suppress occurrence of HHV‐6 viremia and the increase of sIL‐2R levels. HHV‐6 load in the blood was clearly correlated with the serum sIL‐2R level. On the other hand, increased CMV load were found in patients treated with corticosteroids regardless of the start time. The frequency of detection of EBV DNA in peripheral blood was similarly observed in all groups. In conclusion, high‐dose corticosteroids started within 1 week tended to suppress HHV‐6 reactivation through suppression of T cell activation. However, CMV proliferation was promoted by corticosteroids regardless of the start time. These observations suggested that careful consideration should be given to the dose and timing of administration of systemic corticosteroids in the treatment of DIHS/DRESS.
Keywords: cytomegalovirus, drug‐induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms, human herpesvirus 6, soluble interleukin‐2 receptor, systemic corticosteroid
Introduction
Drug‐induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic symptoms (DRESS) is a severe adverse drug reaction, common features of which include widespread skin eruption, high fever and organ involvements, such as hematological abnormalities (leukocytosis, eosinophilia, appearance of atypical lymphocytes), hepatitis and/or renal disturbance, and lymphadenopathy.1, 2 RegiSCAR and the Japanese consensus group have proposed diagnostic criteria for DRESS or DIHS, respectively.1, 2 The RegiSCAR scoring system (DRESS score) classifies DRESS as definite, probable, possible or no case by the symptoms and laboratory findings.2 Almost all DIHS cases diagnosed by the Japanese diagnostic criteria are classified into definite or probable DRESS.
Drug‐induced hypersensitivity syndrome/DRESS is associated with herpesvirus reactivation during the clinical course. Human herpesvirus 6 (HHV‐6) reactivation is exclusively detected approximately 2–3 weeks after the onset of DIHS/DRESS, but not in other severe adverse drug reactions.3, 4 Therefore, in the DIHS diagnostic criteria, the patients lacking HHV‐6 reactivation are diagnosed as atypical DIHS.1 In addition, a sequential reactivation of Epstein–Barr virus (EBV) and/or cytomegalovirus (CMV) is detected in the peripheral blood following HHV‐6.3, 5, 6 In several cases, CMV induces characteristic symptoms such as gastroenteritis, hepatitis, pneumonitis and mucocutaneous ulcer, which affects the outcome of DIHS/DRESS severely.7
Although systemic corticosteroids are known to effectively improve the symptoms of DIHS/DRESS,8, 9 we observed that low‐dose corticosteroids were not fully effective in improving these symptoms and that late‐start corticosteroids sometimes resulted in a prolonged clinical course. Systemic corticosteroids are thought to suppress antiviral immune responses. Ishida et al. 3 reported that corticosteroid therapy for DIHS/DRESS increased HHV‐6 and CMV load, compared with patients treated without corticosteroids. However, the influence of dose and start time of systemic corticosteroids on viral reactivations in DIHS/DRESS has remained unclear. These observations prompted us to analyze the influence of systemic corticosteroids on viral reactivation in DIHS/DRESS, focusing on administration dose and start time.
Methods
Twenty DIHS patients admitted to our hospital between 2002 and 2016 were analyzed. We measured HHV‐6, EBV and CMV viral DNA copy numbers in the blood of these patients using quantitative real‐time polymerase chain reaction4, 10, 11 during the disease course until remission. Serum samples were collected within 1 week after onset (first appearance of skin eruption), at 2, 3 and 4 weeks, and additionally when HHV‐6 DNA was detected in the blood. Serum soluble interleukin‐2 receptor (sIL‐2R) levels were measured by chemiluminescent enzyme immunoassay at SRL (Tokyo, Japan), and thymus and activation‐regulated chemokine (TARC) concentration was measured using an enzyme‐linked immunosorbent assay kit (R&D Systems, Minneapolis, MN, USA). The procedures were approved by the ethics committee of Ehime University Graduate School of Medicine.
To assess the influence of corticosteroid therapy on viral load and clinical course in DIHS, we divided the patients into three groups according to the systemic corticosteroid dose and administration start time. Group A received conservative therapy without systemic corticosteroids. Patients treated with systemic corticosteroids were further divided into two groups. Group B received a high‐dose corticosteroid (prednisolone ≥1 mg/kg per day) within 1 week after the onset of disease. Group C included patients treated with corticosteroid in other ways: either high‐dose corticosteroid but after 1 week of disease onset or low‐dose corticosteroid regardless of administration start time. This is based on the observation that early initiation of high‐dose corticosteroid therapy before reaching a peak of symptoms (2–3 weeks) was effective to improve the disease course.
All data are presented as the mean ± standard error of the mean and were analyzed by Student’s t‐tests for two‐group comparison or by anova followed by Bonferroni’s multiple comparison test. In all analyses, P < 0.05 was taken to indicate statistical significance.
Results
The 20 patients were divided into three groups: group A, seven patients; group B, six patients; and group C, seven patients. The numbers of white blood cells and eosinophils, appearance of atypical lymphocytes, serum alanine transaminase and C‐reactive protein did not differ significantly among the three groups (Table 1). The average period of corticosteroid administration was longer in group C as compared with that in group B (P = 0.025). Relapse of skin rash was frequently observed in group C. The average lengths of hospital stay were 31.3 days (range, 13–48) in group A, 29.5 days (range, 16–49) in group B and 39.3 days (range, 18–81) in group C.
Table 1.
Characteristic of DIHS patients
| Group | Case no. | Age/sex | Culprit drug | Duration between starting of drug and develop of skin eruption | Body temperature (℃) | Lymphadenopathy | Peripheral blood † | ALT † (U/L) | CRP † (mg/dL) | Systemic corticosteroid | Detection of viral DNA in blood (days from onset to initial detection) | Relapsing symptoms or complications over 1 month after the onset | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC (/μL) | Eosinophils (/μL) | Atypical lymphocytes (%) | Initiation day | Dose of prednisolone* (mg/kg per day) | Duration (days) | HHV‐6 | EBV | CMV | ||||||||||
| A | 1 | 54/F | Mexiletine | 30 days | >38 | + | 11 800 | 1530 | 3 | 447 | 1.79 | − | – | – | + (25) | − | − | Hepatitis |
| 2 | 48/F | Phenytoin | 2 years | >39 | + | 15 900 | 7550 | 0 | 146 | 2.96 | – | – | – | + (19) | + (33) | − | Fever, skin rash | |
| 3 | 88/F | Carbamazepine | 50 days | >38 | − | 10 100 | 0 | 0 | 139 | 4.98 | – | – | – | + (16) | + (16) | + (37) | No | |
| 4 | 30/M | Carbamazepine | 39 days | >37 | + | 10 600 | 2600 | 8 | 729 | 3.43 | – | – | – | + (21) | − | + (33) | No | |
| 5 | 64/M | Carbamazepine | 24 days | >37 | − | 10 800 | 430 | 3 | 1521 | 1.3 | – | – | – | + (21) | + (15) | − | No | |
| 6 | 76/F | Carbamazepine | 20 days | >39 | − | 10 400 | 5529 | 9 | 158 | 5.25 | – | – | – | + (18) | − | − | Neurogenic bladder dysfunction, renal impairment | |
| 7 | 32/F | DDS | 21 days | >39 | + | 16 000 | 400 | 44 | 272 | 1.24 | – | – | – | − | − | − | No | |
| Average ± SED | 12 229 ± 850 | 1606 ± 634 | 9.6 ± 5.1 | 487 ± 165 | 3.14 ± 0.54 | |||||||||||||
| B | 8 | 33/F | Zonisamide/Phenobarbital | 31/6 days | >39 | + | 28 300 | 1300 | 26 | 1202 | 4.68 | 4 | 1.1 | 42 | + (14) | − | − | Alopecia areata |
| 9 | 24/F | Phenobarbital | 30 days | >40 | + | 15 900 | 742 | 3 | 275 | 3.38 | 3 | 1.0 | 20 | − | +(13) | − | No | |
| 10 | 42/F | Carbamazepine | 48 days | >38 | + | 14 800 | 2550 | 2.5 | 350 | 0.92 | 5 | 1.1 | 25 | − | − | − | No | |
| 11 | 47/M | Vancomycin | 22 days | >39 | + | 55 800 | 2976 | 0.8 | 528 | 3.22 | 2 | 1.0 | 30 | − | + (14) | + (23) | Fever, urticaria | |
| 12 | 54/M | Sulfamethoxazole/trimethoprim | 35 days | >38 | + | 11 800 | 236 | 24 | 1389 | 6.31 | 4 | 1.3 | 22 | − | + (4 ‡ ) | + (14) | Urticaria | |
| 13 | 63/M | Carbamazepine | 34 days | >39 | + | 17 500 | 835 | 9.5 | 162 | 8.08 | 2 | 0.9 → 1. 1 (day 5) | 28 | + (15) | + (19) | + (41) | Pneumocystis pneumonia, skin rash | |
| Average ± SED | 24 017 ± 5713 | 1607 ± 484 | 11.0 ± 3.8 | 651 ± 178 | 4.43 ± 0.87 | 28 ± 7 | ||||||||||||
| C | 14 | 48/F | Carbamazepine | 2 year | >39 | + | 21 800 | 5777 | 1 | 70 | 7.44 | 13 | 1.1 | 120 | + (20) | + (38) | + (59) | CMV gastroenteritis, skin rash |
| 15 | 44/M | Carbamazepine | 29 days | >39 | + | 22 400 | 5710 | 10 | 282 | 6.21 | 9 | 0.4 → 0.8(day 12) | 50 | + (17) | − | − | Fever, skin rash | |
| 16 | 72/M | Phenytoin | 34 days | >39 | + | 15 000 | 264 | 3 | 22 | 2.69 | 4 | 0.5 | 66 | + (15) | + (28) | + (35) | Pneumocystis pneumonia, skin rash | |
| 17 | 60/M | Zonisamide | 3 months | >39 | + | 13 000 | 1430 | 15 | 1655 | 7.88 | 2 | 0.2 → 1.6 (day 18) | 82 | + (23) | +(19 ‡ ) | +(19 ‡ ) | Skin rash | |
| 18 | 73/F | Mexiletine | 23 days | >39 | + | 29 500 | 2011 | 0 | 114 | 5.85 | 6 | 0.6 → 1.0 (day 13) | 25 | + (13) | + (9) | + (27) | Skin rash | |
| 19 | 30/M | Carbamazepine | 30 days | >39 | + | 13 200 | 0 | 1 | 427 | 9.67 | 11 | 0.8 | 43 | − | + (36) | − | Skin rash | |
| 20 | 40/F | Carbamazepine | 40 days | >40 | + | 15 600 | 2960 | 11 | 213 | 2.9 | 7 | 0.5 | 27 | + (13 ‡ ) | − | +(13 ‡ ) | Skin rash | |
| Average ± SED | 18 643 ± 2008 | 2593 ± 777 | 5.9 ± 2.0 | 397 ± 187 | 6.09 ± 0.84 | 65 ± 31 | ||||||||||||
ALT, alanine aminotransferase; CRP, C‐reactive protein; EBV, Epstein–Barr virus; HHV‐5, human herpesvirus 6; SED, standard error of the mean; WBC, white blood cell.
Body temperature and the most abnormal laboratory data at acute phase are shown.
Viral DNA was detected from initial serum sample.
To assess the disease severity, DRESS score,2 the composite score7 or serum TARC levels were evaluated (Table 2). DRESS score provides a comprehensive disease severity, while the composite score is constructed to evaluate disease severity at any time. Serum TARC level has been used as a parameter to predict the development of DIHS/DRESS and reflects the disease severity.12, 13 The composite scores and serum TARC levels were determined at initial presentation. As a result, DRESS score revealed that two probable cases whose symptoms were milder than those of definite cases were included in group A, but not in group B and C, although no appreciable differences in the composite scores at initial presentation were found between the three groups. On the other hand, TARC levels tended to be higher in group C patients (11 166 ± 4220 pg/mL) than those in group A and group B patients (8213 ± 3104 pg/mL, 7858 ± 3208 pg/mL) without statistical significance.
Table 2.
Assessment of disease severity
| Group | Case no. | DRESS score | Composite score | TARC (pg/mL) (days after onset) | ||
|---|---|---|---|---|---|---|
| Score | Classification † | Score | Severity ‡ | |||
| A | 1 | 8 | Definite | 3 | Moderate | 2820 (6) |
| 2 | 7 | Definite | 7 | Severe | 20 677 (12) | |
| 3 | 4 | Probable | 5 | Severe | 15 317 (14) | |
| 4 | 7 | Definite | 1 | Moderate | 2110 (14) | |
| 5 | 4 | Probable | 3 | Moderate | 1780 (12) | |
| 6 | 8 | Definite | 6 | Severe | 3864 (11) | |
| 7 | 6 | Definite | 1 | Moderate | 10 921 (6) | |
| B | 8 | 7 | Definite | 5 | Severe | 3330 (4) |
| 9 | 7 | Definite | 1 | Moderate | 3330 § (4) | |
| 10 | 8 | Definite | 2 | Moderate | 3136 (4) | |
| 11 | 8 | Definite | 3 | Moderate | 9670 (2) | |
| 12 | 6 | Definite | 2 | Moderate | 1620 (4) | |
| 13 | 7 | Definite | 1 | Moderate | 26 059 § (6) | |
| C | 14 | 8 | Definite | 2 | Moderate | 24 550 (13) |
| 15 | 8 | Definite | 3 | Moderate | 24 600 § (13) | |
| 16 | 6 | Definite | 1 | Moderate | 2660 § (14) | |
| 17 | 7 | Definite | 5 | Severe | 6320 § (19) | |
| 18 | 7 | Definite | 2 | Moderate | 4740 (4) | |
| 19 | 6 | Definite | 6 | Severe | 9486 § (12) | |
| 20 | 8 | Definite | 2 | Moderate | 5803 § (13) | |
DRESS, drug reaction with eosinophilia and systemic symptoms; TARC, thymus and activation‐regulated chemokine.
Score <2, no case; score 2–3, possible case; score 4–5, probable case; score >5, definite case. Definite case is the most severe.
Score <1, mild; score 1–3, moderate; score >4, severe.
Systemic corticosteroids had been administrated before measurement.
In 14 of the 20 patients, HHV‐6 DNA was detected in whole blood at an average of 17.9 days (range, 13–25) after the onset of disease. HHV‐6 reactivation was observed in six of the seven patients in group A and in six of the seven patients in group C (Fig. 1). In contrast, HHV‐6 reactivation was observed in only two of the six patients in group B, with a lower HHV‐6 load without the flaring of symptoms. It is of interest that HHV‐6 was detected for prolonged periods in three patients in group C (cases 14, 17 and 18).
Figure 1.
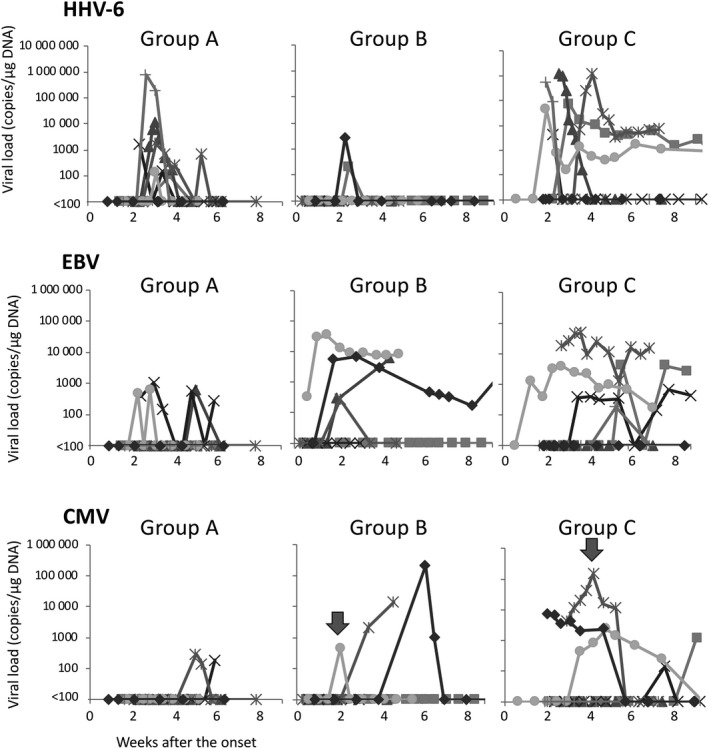
Viral load in the blood. In each group, copy numbers of human herpesvirus 6 (HHV‐6), Epstein–Barr virus (EBV) and cytomegalovirus (CMV) in blood measured by real‐time polymerase chain reaction until disease remission. Arrowheads indicate the time points at which administration of ganciclovir was started.
We evaluated T‐cell activation by measuring sIL‐2R in serum samples, because HHV‐6 proliferation is dependent on T‐cell activation.14 In group A, high sIL‐2R levels observed at 2 weeks decreased with no systemic corticosteroid administration (Fig. 2a). In group B, sIL‐2R levels were significantly lower than in group A and group C at 2 weeks after the onset of disease (Fig. 2b). The pattern of change in sIL‐2R level in group C was similar to that in group A. These findings suggest that early administration of high‐dose corticosteroids effectively suppresses T‐cell activation, but not low dose or late start of corticosteroid administration. We assessed the relationship between sIL‐2R level and HHV‐6 DNA copy number. As shown in Figure 2(c), HHV‐6 DNA copy numbers were correlated with the serum sIL‐2R levels.
Figure 2.
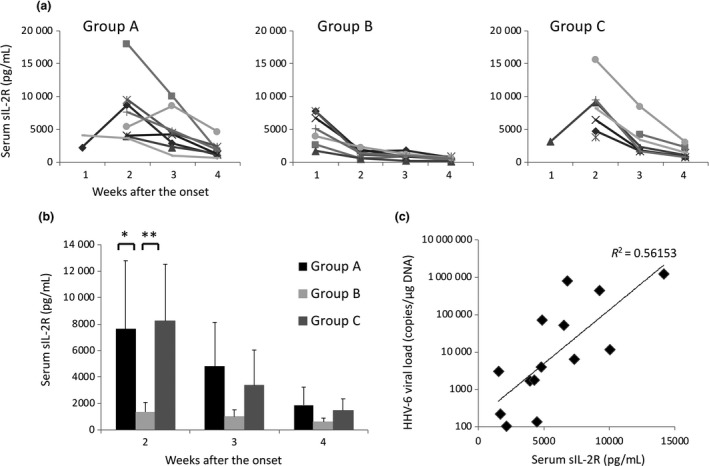
Change of soluble interleukin‐2 receptor (sIL‐2R) during the course of drug‐induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic symptoms (DRESS), and correlation between sIL‐2R levels and human herpesvirus 6 (HHV‐6) load. (a) In each group, sIL‐2R levels were measured within 1 week, and at 2, 3 and 4 weeks after onset of the disease. (b) Means of sIL‐2R levels at 2, 3 and 4 weeks after onset of the disease were compared among each group. *P < 0.05, **P < 0.01. (c) Serum sIL‐2R levels measured when HHV‐6 DNA was detected in the blood were correlated with HHV‐6 load.
Epstein–Barr virus DNA was detected in peripheral whole blood during the disease course at an average of 20.3 days after the onset. It was observed in three patients in group A, four patients in group B and five patients in group C. Although the detection rate of EBV DNA was almost similar among the three groups, the detection period tended to be longer in group B (cases 11 and 13) and group C (cases 14, 17 and 18) (Fig. 1).
Cytomegalovirus DNA was detected in two patients in group A, three patients in group B and five patients in group C at an average of 29.8 days after onset (Fig. 1). In group A, low CMV load was detected with no clinical symptom. In contrast, high CMV load was observed in group B and group C. Case 14 in group C developed skin ulcers and gastric perforation associated with CMV infection. Case 12 (group B) and case 17 (group C) were treated with ganciclovir due to high levels of pp65 antigenemia without clinical symptoms.
Sequential reactivation of HHV‐6, EBV and CMV occurred in one patient of group A, one in group B and four in group C.
Discussion
To assess the disease severity between the three groups, two scoring systems were used.2, 7 The composite score has been recently proposed by Mizukawa et al. 7 to assess the disease severity at any time during the clinical course. It is scored by patient baseline characteristics, clinical manifestation and laboratory data. The scores at initial presentation were not different between the three groups, which ranged from moderate to severe in each group. DRESS score is assessed by clinical manifestation and organ involvement observed throughout the clinical course.2 Although the scores did not differ significantly between the three groups, group A contained two probable cases, but not groups B and C. TARC has been demonstrated as a potential marker of DIHS disease activity and for prediction of HHV‐6 reactivation.12, 13 In group A and group B patients, TARC levels were lower as compared with those of group C. However, it should be noted that initial serum samples from group B patients were obtained within 7 days after the onset. TARC levels reflect mainly the activity of skin rash.12 Case 1 (group A) and case 18 (group C) TARC levels markedly increased from 2820 (day 6) to 19 217 pg/mL (day 13) and from 4740 (day 4) to 32 483 pg/mL (day 9), respectively, in parallel with the exacerbation of skin rash. On the other hand, TARC levels of group B did not increase thereafter (data not shown), which may be due to improvement of skin rash by high‐dose and early‐start corticosteroid therapy. Serum TARC levels and sIL‐2R levels fluctuate similarly during the early acute phase of DIHS/DRESS.12 Taken together, it is considered that milder cases were included in group A. Regardless, HHV‐6 reactivation was frequently observed in group A and group C. CMV reactivation was the most frequently observed in group C. These observations indicate that viral reactivation in DIHS/DRESS is not defined only by the disease severity.
We did not use systemic corticosteroids in group A patients. A systemic corticosteroid is usually used for treatment of DIHS/DRESS, but is not indispensable.15 In two of the seven patients (cases 1 and 7) who visited within 7 days after onset, general condition was markedly improved by discontinuation of causative drugs after admission. In another five patients who consulted our hospital over 10 days after the onset, some improvement in their skin manifestation and systemic symptoms had been observed at initial presentation. Group A yielded information about the natural course of DIHS/DRESS. Symptoms or laboratory findings peaked at 2–3 weeks after onset of the disease without systemic corticosteroid therapy, as reported previously.15 This may be explained by the state of T‐cell activation. High levels of sIL‐2R were observed at 2 weeks after onset, and then decreased thereafter. Following the peak of acute reaction, these patients frequently experienced HHV‐6 viremia, which induced the relapse of fever and/or hepatitis within 1 month after onset, as described in our previous report.4 Moreover, relapses of hepatitis, fever and skin rash, or occurrences of neurogenic bladder dysfunction and renal impairment, were observed in three of these patients after the occurrence of HHV‐6 reactivation. However, gradual improvements and disappearance of symptoms were observed in all patients without corticosteroid. EBV load and low CMV load were detectable in three and two patients without clinical symptoms, respectively.
Interestingly, when a high‐dose corticosteroid was started within 1 week after disease onset, HHV‐6 reactivation tended to be suppressed. Although it remains unclear how HHV‐6 is reactivated from monocytes, reactivated HHV‐6 infects CD4 T cells through CD134,16 a receptor for HHV‐6, and multiplies, resulting in viremia. CD134 is expressed only in activated T cells, and Miyagawa et al. 17 clearly demonstrated that the number of T cells expressing CD134 increased in the acute phase of DIHS/DRESS. Our findings revealed that a high‐dose corticosteroid started within 1 week after onset effectively suppressed T‐cell activation. Under these conditions, reactivated HHV‐6 eventually loses its ability to proliferate after being reactivated from monocytes.
In contrast, low‐dose or late‐start corticosteroids did not suppress T‐cell activation and occurrence of HHV‐6 viremia. Moreover, viral clearance from peripheral whole blood was impaired in three patients (cases 14, 17 and 18), in which high viral load continued for at least 3 weeks without clinical symptoms. Persistent HHV‐6 infection in DIHS patients has been reported, although the clinical significance and the mechanisms remain unclear.18 EBV also tended to be detected for longer periods in the same patients. Antiviral immune responses may be modulated by use of late‐start high‐dose corticosteroids in DIHS/DRESS. We did not examine the viral load after resolution of DIHS/DRESS, so further studies will be necessary to assess the relationship between corticosteroid therapy and persistent HHV‐6 infection.
High CMV load was detected in group B and group C patients treated with corticosteroids. Two patients in group A also had CMV reactivation. However, it should be noted that CMV load in these patients was at a low level. Previous study also confirmed that low CMV load was detected in DIHS/DRESS patients treated without systemic corticosteroids.3 This finding indicates that DIHS/DRESS has the potential to reactivate CMV. It has been demonstrated that regulatory T cells (Treg) are expanded at the acute phase of DIHS.19, 20 Although IL‐2 activates T cells, it coincidentally increases the suppressive activity of Treg based on high‐level expression of high‐affinity IL‐2 receptors on these cells.21 These mechanisms may downregulate excessive T‐cell activation spontaneously, and may also induce further immunosuppression, causing viral reactivation. while, the use of high‐dose systemic corticosteroids is known to enhance the risk of CMV infection due to its immunosuppressive effect in the various conditions.22, 23, 24 Because CMV reactivation is the most important risk factor in the prognosis of DIHS/DRESS,7, greater caution in use of corticosteroids is recommended.
Epstein–Barr virus DNA was detected in peripheral blood during the disease course in 12 of 20 patients. The frequency of detection of EBV DNA in peripheral blood was similarly observed in all groups. However, the detection period tended to be longer in patients treated with corticosteroids. The relationship between EBV DNA detection and clinical symptoms was unclear. In case 9 of group B, low grade fever and cervical lymphadenopathy was observed during the detection of EBV DNA. Increase of EBV DNA in peripheral blood is caused by proliferation of EBV in B cells or by increase of number of B cells in which EBV latent. It may be useful to evaluate the change of anti‐viral capsid antigen immunoglobulin (Ig)G and anti‐diffuse and restricted early antigen IgG, and to analyze lymphocyte subsets of peripheral blood, although we did not examine them. Notably, we find that CMV reactivation is almost preceded by detection of EBV DNA. EBV reactivation may be an indicator and a cause of the reactivation of CMV. Further examination will be needed to discuss the implication of EBV during the disease course of DIHS/DRESS.
In conclusion, our findings suggest that care is required in the dose and timing of administration of systemic corticosteroids for the treatment of DIHS/DRESS. However, the size of our patient population was limited, so further studies are required.
Conflict of Interest
None declared.
Acknowledgment
This study was supported in part by Health and Labor Sciences Research Grants (Research on Intractable Diseases) from the Ministry of Health.
The copyright line for this article was changed on 12 December 2020 after original online publication.
References
- 1. Shiohara T, Iijima M, Ikezawa Z, Hashimoto K. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations. Br J Dermatol 2007; 156: 1083–1084. [DOI] [PubMed] [Google Scholar]
- 2. Kardaun SH, Sekula P, Valeyrie‐Allanore L et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol 2013; 169: 1071–1080. [DOI] [PubMed] [Google Scholar]
- 3. Ishida T, Kano Y, Mizukawa Y, Shiohara T. The dynamics of herpesvirus reactivations during and after severe drug eruptions: Their relation to the clinical phenotype and therapeutic outcome. Allergy Eur J Allergy Clin Immunol 2014; 69: 798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tohyama M, Hashimoto K, Yasukawa M et al. Association of human herpesvirus 6 reactivation with the flaring and severity of drug‐induced hypersensitivity syndrome. Br J Dermatol 2007; 157: 934–940. [DOI] [PubMed] [Google Scholar]
- 5. Kano Y, Hiraharas K, Sakuma K, Shiohara T. Several herpesviruses can reactivate in a severe drug‐induced multiorgan reaction in the same sequential order as in graft‐versus‐host disease. Br J Dermatol 2006; 155: 301–306. [DOI] [PubMed] [Google Scholar]
- 6. Asano Y, Kagawa H, Kano Y, Shiohara T. Cytomegalovirus disease during severe drug eruptions. Arch Dermatol 2009; 145(9): 1030–1036. [DOI] [PubMed] [Google Scholar]
- 7. Mizukawa Y, Hirahara K, Kano Y, Shiohara T. Drug‐induced hypersensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS) severity score: A useful tool for assessing disease severity and predicting fatal cytomegalovirus disease. J Am Acad Dermatol 2019; 80: 670–678. [DOI] [PubMed] [Google Scholar]
- 8. Cacoub P, Musette P, Descamps V et al. The DRESS syndrome: a literature review. Am J Med 2011; 124(7): 588–597. [DOI] [PubMed] [Google Scholar]
- 9. Shiohara T, Kano Y. Drug reaction with eosinophilia and systemic symptoms (DRESS): incidence, pathogenesis and management. Expert Opin Drug Saf 2017; 16: 139–147. [DOI] [PubMed] [Google Scholar]
- 10. Kimura H, Morita M, Yabuta Y et al. Quantitative analysis of Epstein‐Barr virus load by using a real‐time PCR assay. J Clin Microbiol 1999; 37: 132–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tanaka N, Kimura H, Iida K et al. Quantitative analysis of cytomegalovirus load using a real‐time PCR assay. J Med Virol 2000; 60: 455–462. [DOI] [PubMed] [Google Scholar]
- 12. Ogawa K, Morito H, Hasegawa A et al. Identification of thymus and activation‐regulated chemokine (TARC/CCL17) as a potential marker for early indication of disease and prediction of disease activity in drug‐induced hypersensitivity syndrome (DIHS)/drug rash with eosinophilia and systemic symptoms (DRESS). J Dermatol Sci 2013; 69: 38–43. [DOI] [PubMed] [Google Scholar]
- 13. Ogawa K, Morito H, Hasegawa A et al. Elevated serum thymus and activation‐regulated chemokine (TARC/CCL17) relates to reactivation of human herpesvirus 6 in drug reaction with eosinophilia and systemic symptoms (DRESS)/drug‐induced hypersensitivity syndrome (DIHS). Br J Dermatol 2014; 171: 425–427. [DOI] [PubMed] [Google Scholar]
- 14. Frenkel N, Schirmer EC, Katsafanas G, June CH. T‐cell activation is required for efficient replication of human herpesvirus 6. J Virol 1990; 64: 4598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Uhara H, Saiki M, Kawachi S, Ashida A, Oguchi S, Okuyama R. Clinical course of drug‐induced hypersensitivity syndrome treated without systemic corticosteroids. J Eur Acad Dermatology Venereol 2013; 27: 722–726. [DOI] [PubMed] [Google Scholar]
- 16. Tang H, Serada S, Kawabata A et al. CD134 is a cellular receptor specific for human herpesvirus‐6B entry. Proc Natl Acad Sci USA 2013; 110: 9096–9099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miyagawa F, Nakamura Y, Miyashita K et al. Preferential expression of CD134, an HHV‐6 cellular receptor, on CD4 T cells in drug‐induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic symptoms (DRESS). J Dermatol Sci 2016; 83: 151–154. [DOI] [PubMed] [Google Scholar]
- 18. Miyagawa F, Nakamura Y, Ommori R et al. Predominant contribution of CD4 T cells to human herpesvirus 6 (HHV‐6) load in the peripheral blood of patients with drug‐induced hypersensitivity syndrome and persistent HHV‐6 infection. Acta Derm Venereol 2018; 98: 146–148. [DOI] [PubMed] [Google Scholar]
- 19. Takahashi R, Kano Y, Yamazaki Y et al. Defective regulatory T cells in patients with severe drug eruptions: timing of the dysfunction is associated with the pathological phenotype and outcome. J Immunol 2009; 182: 8071–8079. [DOI] [PubMed] [Google Scholar]
- 20. Hanafusa T, Azukizawa H, Matsumura S, Katayama I. The predominant drug‐specific T‐cell population may switch from cytotoxic T cells to regulatory T cells during the course of anticonvulsant‐induced hypersensitivity. J Dermatol Sci 2012; 65: 213–219. [DOI] [PubMed] [Google Scholar]
- 21. Spolski R, Li P, Leonard WJ. Biology and regulation of IL‐2: from molecular mechanisms to human therapy. Nat Rev Immunol 2018; 18: 648–659. [DOI] [PubMed] [Google Scholar]
- 22. Emery VC, Sabin CA, Cope AV, Gor D, Hassan‐Walker AF, Griffiths PD. Application of viral‐load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet 2000; 355: 2032–2036. [DOI] [PubMed] [Google Scholar]
- 23. Hakki M, Riddell SR, Storek J et al. Immune reconstitution to cytomegalovirus after allogeneic hematopoietic stem cell transplantation: impact of host factors, drug therapy, and subclinical reactivation. Blood 2003; 102: 3060–3067. [DOI] [PubMed] [Google Scholar]
- 24. Yamashita M, Ishii T, Iwama N et al. Incidence and clinical features of cytomegalovirus infection diagnosed by cytomegalovirus pp65 antigenemia assay during high dose corticosteroid therapy for collagen vascular diseases. Clin Exp Rheumatol 2006; 24: 649–655. [PubMed] [Google Scholar]


