Summary
Background
For many years dermatologists have had access to few therapies for patients with moderate‐to‐severe atopic eczema (AE). New promising therapies are entering the market but conventional phototherapies and systemic therapies have more well‐known safety profiles, lower costs and wider availability.
Objectives
To provide insight into current prescribing practices of conventional phototherapy and systemic immunomodulatory therapies for adults with chronic AE, and the factors influencing these prescribing practices, before biologics and other novel therapeutics become routine clinical practice.
Methods
In this exploratory study dermatologists were invited to participate in an online survey via a mailing list of the European Academy of Dermatology and Venereology and national societies. Data were collected on participant characteristics (including clinical practice data), the use of phototherapies and systemic therapies, and factors influencing their use.
Results
From 30 European countries, 238 out of 361 dermatologists willing to participate (65·9%) completed the survey, with 229 meeting the inclusion criteria. For phototherapy (prescribed by 84·7%), most preferred narrowband ultraviolet B as first line (80·9%) and psoralen plus ultraviolet A as second (21·6%). For systemic therapy (prescribed by 95·2%) ciclosporin (54·1%), oral corticosteroids (32·6%) and methotrexate (30·7%) were used first line. Dermatologists relied mostly on personal experience for prescribing phototherapy and systemic therapy. Azathioprine and mycophenolic acid were prescribed by only 135 (59·0%) and 85 (37·1%) participants in total, mostly due to a lack of personal experience.
Conclusions
This study provides insight into prescribing practices for conventional phototherapy and systemic therapy in Europe and shows that off‐label therapies are also preferred as first‐line choice of systemic therapy.
Short abstract
What is already known about this topic?
Varying prescribing practices were found for adult (in the UK) and paediatric (in Northern America and Europe) patients with moderate‐to-severe atopic eczema (AE).
Not much is known about the prescription of phototherapy and (off‐label) systemic therapy for adult patients in Europe.
Although therapies like dupilumab are promising new treatment modalities, better‐known safety profiles, lower costs and better availability are reasons to improve the evidence profile of conventional systemic therapies like ciclosporin.
What does this study add?
Prescribing practices of European dermatologists treating adult patients with moderate‐to-severe AE show diversity.
Most dermatologists prefer narrowband ultraviolet B as first‐line phototherapy, followed by psoralen plus ultraviolet A as second line.
Next to ciclosporin, which is most commonly prescribed, (off‐label) methotrexate and oral corticosteroids are also frequently used as first‐line systemic agents in chronic AE.
Lack of personal experience with azathioprine and mycophenolic acid was the most important reason against their prescription.
What are the clinical implications of the work?
The results from this study might help to improve the experience with, and prescribing of, all available conventional phototherapies and (off‐label) systemic therapies.
Guidelines developers might use these results to develop and implement treatment algorithms.
Linked Comment: Bruin‐Weller. Br J Dermatol 2020; 183:987–988.
Plain language summary available online
For years dermatologists have had a small array of available therapies for patients with moderate‐to‐severe atopic eczema (AE) – but no robust guidance for these therapies, as the supporting evidence has been of varying quality and only a few long‐term studies are available.1, 2, 3, 4, 5 Currently, the first biologic with a promising efficacy profile, dupilumab, is registered in Europe and the USA for adult and adolescent patients with AE.6, 7, 8 However, long‐term safety evidence is missing.9 Conventional systemic therapies like ciclosporin have more well‐known side‐effect profiles10 lower costs, and will probably be more accessible to a wide group of patients with AE.11 We therefore still see a need to pursue a better evidence profile for conventional phototherapies and systemic therapies.
Survey studies investigating the prescribing practices of conventional phototherapies and systemic therapies of the TREatment of ATopic eczema (TREAT) Registry Taskforce initiative (https://treat-registry-taskforce.org) have been performed in paediatric patients with AE in Europe and Northern America12, 13 and in adult patients with AE in the UK.14 These studies have shown varying prescribing practices and varying factors influencing these prescribing practices among dermatologists. As the arrival of new biologics will probably influence prescribing practices, it is important to determine the current treatment approaches and reasons for or against prescribing therapies for adult patients with AE in continental Europe.
This study will contribute to a clearer view of the usage and prescribing practices of conventional phototherapies and systemic therapies, and of factors for or against prescribing certain treatments. Ultimately this might aid in the development of better evidence profiles for these therapies and more uniform treatment algorithms.
Materials and methods
Study design
We conducted an online, anonymous, multiple‐response survey among European dermatologists caring for adult patients with moderate‐to‐severe AE. Moderate‐to‐severe AE was defined as AE that is not adequately controlled by standard and optimized topical treatment. Participants were asked to make decisions for patients who did not have an acute flare.
The survey was developed in Snap Surveys software (https://www.snapsurveys.com), was available in the English language and was pilot tested. As this study is not part of the Medical Research Involving Human Subjects Act (WMO), a medical ethics committee was not consulted. The survey was live from 5 March 2018 to 28 September 2018. In total five reminders were sent.
Survey questionnaire
Both the protocol for and the questions used in the survey were based on previous surveys performed by Proudfoot et al.,12 Totri et al.13 and Taylor et al.14 and were slightly adapted. The survey consisted of 135 questions in total, spread over five different sections: ‘demographics’, ‘treatment options’, ‘phototherapy’, ‘systemic therapy’ and ‘future work’.
The collected demographic data of the respondents can be found in Table 1. In the treatment options section, participants were queried about their first‐, second‐ and third‐line choices of therapy, assuming the scenario that daycare treatment (intensive topical therapy, potentially combined with bathing therapy, psychosocial support, education and phototherapy, two to three times a week), hospital admission, phototherapy and systemic therapy were available. Participants were also asked which treatments were available at their centre.
Table 1.
Participant characteristics (including professional characteristics) (n = 229)
| Characteristic | n (%) |
|---|---|
| Age (years) | |
| 20–30 | 16 (7·0) |
| 31–40 | 54 (23·6) |
| 41–50 | 66 (28·8) |
| 51–60 | 67 (29·3) |
| > 60 | 26 (11·4) |
| Sex | |
| Female | 135 (59·0) |
| Male | 94 (41·0) |
| Type of workplace | |
| Community | 1 (0·4) |
| General hospital | 38 (16·6) |
| Private practice | 42 (18·3) |
| University teaching hospital | 148 (64·6) |
| Years of experience | |
| 0–4 | 14 (6·1) |
| 5–10 | 45 (19·7) |
| 11–20 | 70 (30·6) |
| > 20 | 100 (43·7) |
| Number of treated patients per 3 months | |
| < 10 | 30 (13·1) |
| 10–50 | 116 (50·7) |
| 51–100 | 58 (25·3) |
| 101–200 | 17 (7·4) |
| > 200 | 8 (3·5) |
| Patients with moderate‐to‐severe AE in the population | |
| < 5% | 34 (14·8) |
| 5–10% | 59 (25·8) |
| 11–25% | 55 (24·0) |
| 26–40% | 31 (13·5) |
| 41–60% | 24 (10·5) |
| > 60% | 26 (11·4) |
| Number of initiated patients on photo‐ or systemic therapy per 3 months | |
| 0 | 1 (0·4) |
| 1–5 | 109 (47·6) |
| 6–10 | 62 (27·1) |
| 11–20 | 35 (15·3) |
| > 20 | 22 (9·6) |
| Ethnicity of the majority of patientsa | |
| White | 225 (98·3) |
| Ethnicities of patients seen occasionallya | |
| Asian Chinese | 174 (76·0) |
| Black African/Afro‐Caribbean | 161 (70·3) |
| South Asian | 158 (69·0) |
| Asian – other | 142 (62·0) |
| Hispanic/Latino | 139 (60·7) |
| Five most frequently used measurement instrumentsa | |
| SCORing Atopic Dermatitis | 155 (67·7) |
| Dermatology Life Quality Index | 129 (56·3) |
| Eczema Area and Severity Index | 125 (54·6) |
| Investigator's Global Assessment | 77 (33·6) |
| Visual analogue scale for itch | 53 (23·1) |
AE, atopic eczema. aParticipants were able to select multiple options.
Subsequently, for phototherapy and systemic therapy, participants were asked if they prescribed the treatments and, if so, how often. If participants did not prescribe phototherapy and/or systemic therapy they were directed to the next section or to the end of the survey.
Questions were then asked about first‐, second‐ and third‐line choices of therapy. In the phototherapy section several options were offered: broadband ultraviolet B (BB‐UVB), narrowband ultraviolet B (NB‐UVB), ultraviolet A plus ultraviolet B (UVAB), psoralen plus ultraviolet A (PUVA), ultraviolet A (UVA) and ultraviolet A1 (UVA1). In the systemic therapy section the options were ciclosporin, azathioprine, methotrexate, mycophenolic acid, oral corticosteroids and ‘other’. For all of the questions on first‐, second‐ and third‐line choices participants were able to select multiple therapies. Participants were queried about different subgroups (e.g. patients with relative contraindications, pregnant patients or elderly patients) in which they prescribed certain systemic therapies. In addition, for each treatment option participants were asked about the dosing, duration of treatment, discontinuation regimens and reasons for or against prescribing a therapy (for phototherapy overall, and for systemic therapy overall and per treatment; Tables S1–S6; see Supporting Information). Finally, participants had the opportunity to highlight current gaps in knowledge.
Participants
Eligible participants were (resident) dermatologists in Europe. As Taylor et al. had already performed a survey among UK dermatologists treating adult patients with AE,14 (resident) dermatologists from the UK were excluded. A mailing list of European Academy of Dermatology and Venereology members and 41 national societies (Table S7; see Supporting Information) was used to distribute an invitation to register for participation in the survey. Those who were willing to participate were asked to register with their email address, after which they received a personal link to the survey. To avoid duplicate answers from the same participant, the list of all email addresses was screened manually and duplicates were removed.
Statistical analyses
Descriptive statistics were primarily used. To identify differences in participant characteristics and choice of systemic therapy, a χ2‐test for categorical variables was used. P‐values < 0·05 were considered statistically significant. The statistical analyses were conducted using SPSS 25 (IBM, Armonk, NY, USA) and R Statistical Software version 3·50 (R Foundation, Vienna, Austria).
Results
Study population
In total, 361 (resident) dermatologists registered for participation in the survey. Of these, 238 completed the survey (65·9%). Four participants were excluded due to being based in the UK, four for not prescribing phototherapy or systemic therapy and one for being a general practitioner. The participants originated from 30 different countries (Figure 1). Of the remaining 229 participants, 135 (59·0%) were female and the majority worked in a university teaching hospital (148 of 229, 64·6%). In total, 100 participants (43·7%) had treated patients with AE for over 20 years and 70 (30·6%) for 11–20 years, indicating that the majority had a significant amount of expertise. An overview of the participant and professional characteristics is given in Table 1.
Figure 1.
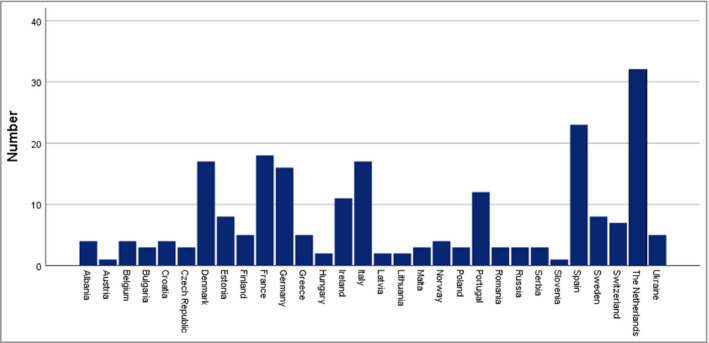
Number of participants per country.
General prescribing behaviour
The most common first‐line therapy in general reported for patients with moderate‐to‐severe AE was photo(chemo)therapy, prescribed by 95 participants (41·5%), followed by daycare therapy (90 of 229, 39·3%) and systemic therapy (61 of 229, 26·6%). As second line, systemic therapy (114 of 229, 49·8%) and photo(chemo)therapy (88 of 229, 38·4%) were mostly selected. An overview of the availability of phototherapy and systemic therapies for participating countries can be found in Figure 2. When queried about the most relevant studies for the treatment of moderate‐to‐severe AE, participants indicated that especially long‐term safety, long‐term control and efficacy, and head‐to‐head trials are of importance. Regarding gaps in the evidence base, participants mentioned education of patients; data on the efficacy, safety and duration of treatment of current conventional systemic therapies; data for personalized medicine using biomarkers; and treatment of itch specifically.
Figure 2.
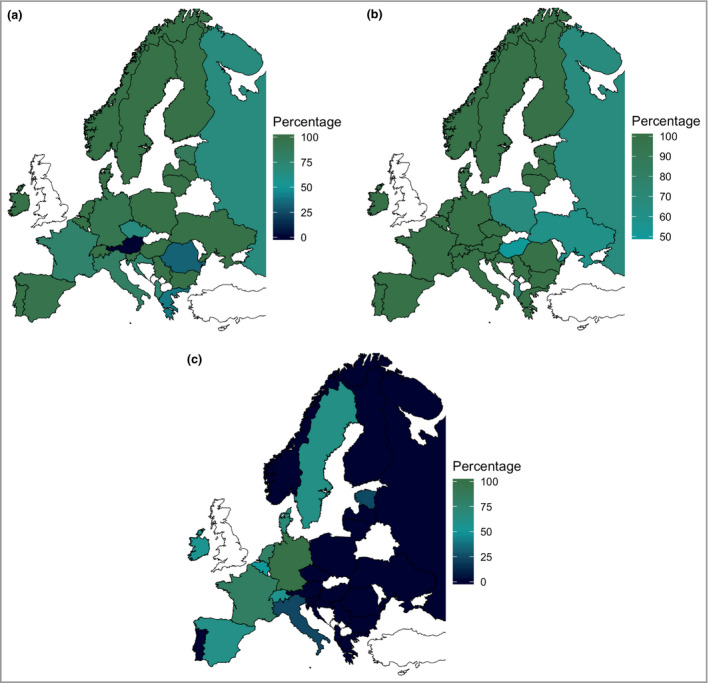
(a) Percentages of participants who had access to photo(chemo)therapy (in general) per country. (b) Percentages of participants who had access to systemic therapy (in general) per country. (c) Percentages of participants who had access to dupilumab per country. The countries with more than 10 participants are Denmark, France, Germany, Ireland, Italy, Portugal, Spain and the Netherlands.
Phototherapy
Of all participants, 194 (84·7%) indicated using phototherapy for adult patients with AE. Of these participants, 32 (16·5%) indicated that they primarily prescribe phototherapy, 59 (30·4%) indicated they use this often, 64 (33·0%) sometimes and 39 (20·1%) rarely. The most commonly prescribed first‐line UV therapy was NB‐UVB, by 157 participants (80·9%), followed by BB‐UVB (15 of 194, 7·7%) and UVAB (12 of 194, 6·2%). PUVA was most frequently prescribed as second‐line UV treatment (42 of 194, 21·6%), followed by BB‐UVB (32 of 194, 16·5%) and UVAB (28 of 194, 14·4%). PUVA (25 of 194, 12·9%) and UVA1 (nine of 194, 4·6%) were most commonly selected as the third‐line choice of UV therapy (Figure 3a). The most important reason for the use of phototherapy was personal experience with the treatment (114 of 194, 58·8%) and the most important reason against was the unavailability of phototherapy at a centre (16 of 35, 46%) (Tables S1 and S2; see Supporting Information).
Figure 3.
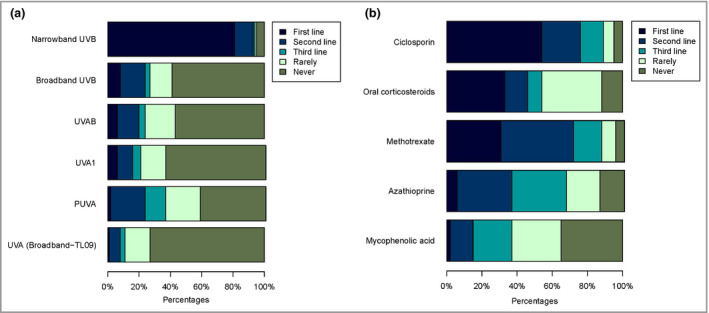
First‐, second‐ and third‐line choice of (a) phototherapy (n = 194) and (b) systemic therapy (n = 218). Participants were able to select multiple therapies as first line, second line, third line, rarely or never prescribed.
Systemic therapy and dosing schedules
In total 218 participants (95·2%) indicated they prescribe systemic therapy. Several treatments were prescribed as the first‐line choice of therapy: ciclosporin by 118 (54·1%), oral corticosteroids by 71 (32·6%) and methotrexate by 67 (30·7%). Azathioprine and mycophenolic acid were only prescribed first line by 12 (5·5%) and four (1·8%) participants. Methotrexate (89 of 218, 40·8%), azathioprine (67 of 218, 30·7%) and ciclosporin (49 of 218, 22·5%) were the most commonly prescribed second‐line treatments. Azathioprine (67 of 218, 30·7%), mycophenolic acid (48 of 218, 22·0%) and methotrexate (34 of 218, 15·6%) were the most commonly selected third‐line treatments (Figure 3b).
First‐line methotrexate prescription was highest in university teaching hospitals (38%, compared with 18% for general hospital and 15% for private practice), while prescription as third line was more frequent in general hospitals (24%, compared with 12% and 15% for private practice and university hospitals, respectively). In addition, private practices prescribed less methotrexate than the other workplaces (P = 0·0053; Table S8c; see Supporting Information). Mycophenolic acid was prescribed mostly by university‐based dermatologists (15% and 28% for second and third line, respectively). Dermatologists from general hospitals and private practices rarely (39% and 18%) or never (39% and 65%) prescribed mycophenolic acid, respectively (P < 0·001; Table S8d; see Supporting Information). There were no significant differences between the type of workplace and the prescription patterns of the other systemic therapies (Table S8a, b, e; see Supporting Information).
The following other demographic variables significantly affected the responses. Less experienced participants more frequently prescribed mycophenolic acid first and third line. Mycophenolic acid was more frequently prescribed second and third line when more patients were initiated on phototherapy and systemic therapy (per 3 months). Female participants prescribed less mycophenolic acid. Participants prescribed oral corticosteroids more as first‐, second‐ or third‐line therapy when they treated fewer patients with moderate‐to‐severe AE. Azathioprine was most frequently prescribed second and third line in participants treating 10–200 patients per 3 months, but rarely or never when < 10 or > 200 patients were treated. Younger participants prescribed methotrexate more first and second line, while older participants did this rarely or never.
First‐, second‐ and third‐line choices of systemic therapy grouped by country (only those with > 10 participants) are presented in Tables S9–11 (see Supporting Information). These tables show that dermatologists in Denmark and Ireland more often prescribe methotrexate as first line (88% and 91%, respectively, vs. 30·7% on average); that dermatologists in the Netherlands more often prescribe ciclosporin as first line (87% vs. 54·1% on average) and that oral corticosteroids are mostly prescribed first line in Spain (57% vs. 32·6% on average). Table 2 shows prescribing practices in subgroups of patients. Oral corticosteroids and ciclosporin are prescribed to both pregnant (110, 50·5% and 64, 29·4%, respectively) and lactating patients (102, 46·8% and 41, 18·8%, respectively). Methotrexate was the preferred therapy for patients with comorbidities (78 of 218, 35·8%) and elderly patients (106 of 218, 48·6%).
Table 2.
Prescription of systemic medication in subgroups (218 participants)
| Ciclosporin | Azathioprine | Methotrexate | Mycophenolic acid | Oral corticosteroids | |
|---|---|---|---|---|---|
| Patients with relative contraindications | 44 (20·2) | 21 (9·6) | 45 (20·6) | 28 (12·8) | 66 (30·3) |
| Pregnant patients | 64 (29·4) | 8 (3·7) | 2 (0·9) | 2 (0·9) | 110 (50·5) |
| Lactating patients | 41 (18·8) | 7 (3·2) | 2 (0·9) | 2 (0·9) | 102 (46·8) |
| Obese patients | 72 (33·0) | 49 (22·5) | 84 (38·5) | 31 (14·2) | 25 (11·5) |
| Teenagers | 127 (58·3) | 37 (17·0) | 63 (28·9) | 26 (11·9) | 41 (18·8) |
| Patients with malignant disease (current or history) | 16 (7·3) | 7 (3·2) | 74 (33·9) | 7 (3·2) | 82 (37·6) |
| Patients with comorbidities | 36 (16·5) | 35 (16·1) | 78 (35·8) | 33 (15·1) | 40 (18·3) |
| Elderly | 34 (15·6) | 44 (20·2) | 106 (48·6) | 27 (12·4) | 39 (17·9) |
The data are presented as n (%). This table shows only in which subgroups participants would prescribe certain therapies. Relative and absolute contraindications such as pregnancy and lactation during methotrexate use are not shown but should always be considered when making a choice of systemic therapy.
Not all systemic therapies were prescribed by all participants. Ciclosporin was prescribed by 201 participants (87·8%), methotrexate by 199 (86·9%), oral corticosteroids by 184 (80·3%), azathioprine by 135 (59·0%) and mycophenolic acid by only 85 (37·1%). Tables 3, 4, 5 provide information on the frequency of use and discontinuation regimens, the initial and maximum dosing schedules and the average and maximum durations on therapy. ‘Other’ therapies used were ‘antibiotics’ (not specified), omalizumab, rituximab, alitretinoin, photopheresis, dupilumab, ‘study medication’ (not specified), intravenous immunoglobulin, tacrolimus and tofacitinib.
Table 3.
Frequency of use and discontinuation regimens of systemic therapy
| Ciclosporin | Azathioprine | Methotrexate | Mycophenolic acid | Oral corticosteroids | |
|---|---|---|---|---|---|
| n = 201 | n = 135 | n = 199 | n = 85 | n = 184 | |
| Frequency of use | |||||
| Rarely (< 10%) | 72 (35·8) | 82 (60·7) | 79 (39·7) | 70 (82) | 80 (43·5) |
| Sometimes (11–25%) | 70 (34·8) | 44 (32·6) | 54 (27·1) | 14 (16) | 58 (31·5) |
| Often (26–50%) | 48 (23·9) | 9 (6·7) | 41 (20·6) | 1 (1) | 38 (20·7) |
| Mostly (> 50%) | 11 (5·5) | 0 (0·0) | 25 (12·6) | 0 (0) | 8 (4·3) |
| Discontinuation regimen | |||||
| Titrate dose over 1 week | 12 (6·0) | 5 (3·7) | 5 (2·5) | 5 (6) | 52 (28·3) |
| Titrate dose over 1 month | 80 (39·8) | 47 (34·8) | 46 (23·1) | 24 (28) | 76 (41·3) |
| Halve dose every 2 weeks | 47 (23·4) | 27 (20·0) | 36 (18·1) | 20 (24) | 33 (17·9) |
| Discontinue without titration | 50 (24·9) | 50 (37·0) | 94 (47·2) | 33 (39) | 15 (8·2) |
The data are presented as n (%).
Table 4.
Initial and maximum dosages of systemic therapy
| Ciclosporin | Azathioprine | Methotrexate | Mycophenolic acid | Oral corticosteroids | |||||
|---|---|---|---|---|---|---|---|---|---|
| n = 201 | n = 135 | n = 199 | n = 85 | n = 184 | |||||
| mg kg−1 per day | mg kg−1 per day | mg per week | g per day | mg kg−1 per day | |||||
| Initial dose | |||||||||
| Dose | n (%) | Dose | n (%) | Dose | n (%) | Dose | n (%) | Dose | n (%) |
| < 2·5 | 22 (10·9) | < 1 | 20 (14·8) | < 5 | 6 (3·0) | < 1 | 11 (13) | < 0·5 | 75 (40·8) |
| 2·5–3·5 | 110 (54·7) | 1–2 | 98 (72·6) | 5–10 | 60 (30·2) | 1–1·5 | 40 (47) | 0·5–1·0 | 107 (58·2) |
| 3·6–4·5 | 37 (18·4) | 2·1–3 | 17 (12·6) | 11–15 | 109 (54·8) | 1·6–2 | 32 (38) | > 1·0 | 2 (1·1) |
| 4·6–5·0 | 32 (15·9) | > 3 | 0 (0·0) | 16–20 | 22 (11·1) | > 2 | 2 (2) | ||
| > 5·0 | 0 (0·0) | 21–25 | 2 (1·0) | ||||||
| > 25 | 0 (0·0) | ||||||||
| Maximum dose | |||||||||
| Dose | n (%) | Dose | n (%) | Dose | n (%) | Dose | n (%) | Dose | n (%) |
| < 2·5 | 2 (1·0) | < 1 | 2 (1·5) | < 5 | 1 (0·5) | < 1 | 2 (2) | < 0·5 | 24 (13·0) |
| 2·5–3·5 | 32 (15·9) | 1–2 | 53 (39·3) | 5–10 | 8 (4·0) | 1–1·5 | 12 (14) | 0·5–1·0 | 146 (79·3) |
| 3·6–4·5 | 42 (20·9) | 2·1–3 | 76 (56·3) | 11–15 | 33 (16·6) | 1·6–2 | 51 (60) | > 1·0 | 14 (7·6) |
| 4·6–5·0 | 120 (59·7) | > 3 | 4 (3·0) | 16–20 | 69 (34·7) | > 2 | 20 (24) | ||
| > 5·0 | 5 (2·5) | 21–25 | 87 (43·7) | ||||||
| > 25 | 1 (0·5) | ||||||||
Table 5.
Average and maximum durations of use of systemic therapy
| Ciclosporin | Azathioprine | Methotrexate | Mycophenolic acid | Oral corticosteroids | |||||
|---|---|---|---|---|---|---|---|---|---|
| n = 201 | n = 135 | n = 199 | n = 85 | n = 184 | |||||
| Average duration (months) | |||||||||
| Duration | n (%) | Duration | n (%) | Duration | n (%) | Duration | n (%) | Duration | n (%) |
| 0–3 | 21 (10·4) | 0–3 | 12 (8·9) | 0–3 | 13 (6·5) | 0–3 | 3 (4) | < 1 | 143 (77·7) |
| 4–6 | 86 (42·8) | 4–6 | 15 (11·1) | 4–6 | 25 (12·6) | 4–6 | 10 (12) | 1–3 | 36 (19·6) |
| 7–12 | 61 (30·3) | 7–12 | 43 (31·9) | 7–12 | 47 (23·6) | 7–12 | 31 (36) | 4–6 | 4 (2·2) |
| 13–18 | 21 (10·4) | 13–18 | 27 (20·0) | 13–18 | 36 (18·1) | 13–18 | 17 (20) | 7–12 | 1 (0·5) |
| 19–24 | 10 (5·0) | 19–24 | 22 (16·3) | 19–24 | 34 (17·1) | 19–24 | 11 (13) | 13–18 | 0 (0·0) |
| > 24 | 2 (1·0) | > 24 | 16 (11·9) | > 24 | 44 (22·1) | > 24 | 13 (15) | 19–24 | 0 (0·0) |
| > 24 | 0 (0·0) | ||||||||
| Maximum duration (months) | |||||||||
| Duration | n (%) | Duration | n (%) | Duration | n (%) | Duration | n (%) | Duration | n (%) |
| 0–3 | 3 (1·5) | 0–3 | 2 (1·5) | 0–3 | 3 (1·5) | 0–3 | 2 (2) | < 1 | 74 (40·2) |
| 4–6 | 27 (13·4) | 4–6 | 12 (8·9) | 4–6 | 14 (7·0) | 4–6 | 2 (2) | 1–3 | 80 (43·5) |
| 7–12 | 64 (31·8) | 7–12 | 17 (12·6) | 7–12 | 23 (11·6) | 7–12 | 13 (15) | 4–6 | 24 (13·0) |
| 13–18 | 25 (12·4) | 13–18 | 10 (7·4) | 13–18 | 16 (8·0) | 13–18 | 8 (9) | 7–12 | 3 (1·6) |
| 19–24 | 55 (27·4) | 19–24 | 27 (20·0) | 19–24 | 15 (7·5) | 19–24 | 13 (15) | 13–18 | 0 (0·0) |
| > 24 | 27 (13·4) | > 24 | 67 (49·6) | > 24 | 128 (64·3) | > 24 | 47 (55) | 19–24 | 1 (0·5) |
| > 24 | 2 (1·1) | ||||||||
Of the 199 participants prescribing methotrexate, 100 (50·3%) indicated never giving a test dose, while 36 (18·1%) indicated giving a test dose of 7·5 mg, 23 (11·6%) of 10 mg, 21 (10·6%) of 5 mg and seven (3·5%) of 2·5 mg. Oral corticosteroids were given for an acute flare (179 of 184, 97·3%), during initiation of other immunosuppression (73 of 184, 39·7%), in addition to other immunosuppression (23 of 184, 12·5%) and only rarely as ongoing treatment (six of 184, 3·3%). In general, participants indicated that especially their own clinical experience (140 of 218, 64·2%), baseline tests and comorbidities (128 of 218, 58·7%), a low potential long‐term side‐effect profile (123 of 218, 56·4%), sufficient medium‐term (> 3 months) efficacy (118 of 218, 54·1%) and their knowledge of national or international guidelines (115 of 218, 52·8%) influenced their choice for systemic therapy (Table S3; see Supporting Information).
Regarding reasons for prescribing methotrexate, dermatologists in Denmark (17 in total) and Ireland (11 in total) mainly indicated that better long‐term efficacy (14 of 17, 82% and 10 of 11, 91%, respectively); a low potential acute (eight of 17, 47% and seven of 11, 64%) and long‐term (12 of 17, 71% and four of 11, 36%) side‐effect profile; knowledge of international guidelines (10 of 17, 59% and four of 11, 36%), knowledge of expert opinions (six of 17, 35% and eight of 11, 73%) and personal experience with the treatment (10 of 17, 59% and 10 of 11, 91%) were reasons for their prescription of methotrexate.
Perceived barriers for prescribing systemic therapy (not prescribed by 11 participants) were a high potential acute side‐effect profile (six of 11, 55%), a suspected risk of long‐term organ toxicity (five of 11, 45%), comorbidities (five of 11, 45%) and patient preferences (five of 11, 45%) (Table S4; see Supporting Information). Both azathioprine (not prescribed by 83 participants) and mycophenolic acid (not prescribed by 133 participants) were rarely prescribed as the first‐line choice, due mainly to a lack of personal experience (50 of 83, 60% and 78 of 133, 58·6%, respectively). Other reasons for and against prescribing specific systemic therapies are given in Tables S5 and S6 (see Supporting Information).
Discussion
The prescribing of phototherapy and systemic immunomodulatory treatments for AE varies across European countries. Despite the fact that clinical experience seemed the most relevant reason for or against prescribing certain therapies, the majority of dermatologists seem to prescribe treatments according to current guidelines.10, 15, 16, 17, 18
In this study NB‐UVB and PUVA were found to be the first‐ and second‐line choices of therapies for photo(chemo)therapy. This corresponds with results from the UK TREAT adult survey,14 but only partly with the recommendations that can be found in guidelines. Garritsen et al.2 found that NB‐UVB and UVA1 appear to be the most effective phototherapies based on the available evidence. In our study UVA1 was only prescribed by a small minority, but this could be based on a low availability of UVA1 for our participants. A more recent study by Ling et al. showed that if NB‐UVB is not effective in severe AE, PUVA therapy may also be considered.19
Ciclosporin was found to be the preferred first‐line systemic therapy, which is in line with multiple guidelines and both the European and North American paediatric TREAT surveys.12, 13, 15, 17, 18 However, it does not correspond with the results of the UK adult treatment survey, in which azathioprine was preferred as first‐line systemic therapy.14 A large proportion of dermatologists did not favour ciclosporin as first‐line therapy and preferred oral corticosteroids (32·6%) or methotrexate (30·7%). This is interesting, as during the course of our study ciclosporin was the only licensed systemic agent for AE in Europe.20
As second‐line treatment methotrexate and azathioprine were most frequently selected. In most guidelines no specific second‐line therapy is suggested, but recommendations are made that methotrexate, azathioprine and mycophenolic acid are options to consider when ciclosporin fails to achieve results in the treatment of AE.15, 17, 18
No significant difference was found between the type of workplace and prescription patterns for ciclosporin, azathioprine and oral corticosteroids. For ciclosporin this could be explained by the participants’ knowledge of guidelines (as first‐choice therapy) and the wider evidence base for ciclosporin (Table S5; see Supporting Information). For oral corticosteroids this might be explained by their historically very regular prescription by both academic and nonacademic dermatologists.
Another interesting finding is that the majority of dermatologists prescribed ciclosporin for a maximum period of 18 months, while in daily practice some patients seem to tolerate ciclosporin longer.21 Furthermore, the prescription of ciclosporin and oral corticosteroids for pregnant or lactating patients is in accordance with the recent position paper from the European Task Force on Atopic Dermatitis (ETFAD) on treatment of parental AE during pregnancy and lactation.22 Oral corticosteroids were found to be most frequently prescribed for an acute flare (97·3%) or during initiation of other immunosuppression (39·7%), which corresponds with a recent consensus paper on systemic corticosteroids published by the International Eczema Council.23 However, it is not in line with the fact that 32·6% of the participants prescribe oral corticosteroids as first‐line therapy in the case of patients with moderate‐to‐severe AE who did not have an acute flare.
When questioned about methotrexate, almost half of the participants indicated using a test dose before starting therapy, while the ETFAD and American Academy of Dermatology guidelines do not mention the use of a test dose.10, 17 Methotrexate was further the most preferred therapy for elderly patients and those with comorbidities. This might be explained by the fact that dermatologists historically have broad experience with the prescription of methotrexate in other skin diseases – such as psoriasis – and in different subgroups of patients. Also, methotrexate has a lower acute toxicity risk than ciclosporin, for example, and could be suitable for long‐term use.
In total 229 completed surveys from 30 countries were analysed in this study, providing a representation of practice within Europe (64·6% academic dermatologists, 35·4% nonacademic dermatologists). As data were collected on both phototherapies and systemic therapies, this study provides a complete view on the treatment of patients with moderate‐to‐severe AE in Europe before the standard use of biologics for AE–although the fact that the majority of the countries had fewer than five participants might have influenced the results. In this study only dermatologists who treated patients with moderate‐to‐severe AE regularly were included. Selection bias may play a role in our study. The majority of dermatologists in our study were based in academic hospitals, participants were not queried about the simultaneous use of two systemic therapies and recall bias could play a role. Our intercountry data show some interesting differences between countries. However, these results need to be interpreted carefully as more participants per country are needed for reliable intercountry analyses.
The results from this study provide evidence that next to (on‐label) ciclosporin, phototherapies and off‐label systemic therapies are chosen by dermatologists as the first choice of treatment for patients with moderate‐to‐severe AE. This is yet another reason to refine the guidance provided to dermatologists treating adult patients with AE. More high‐quality evidence is needed. Large, well‐designed prospective patient cohorts (for real‐life data) or (living) network meta‐analyses24 might provide the data that are needed, and might provide guidance on the treatment of subgroups, for example. The TREAT Registry Taskforce25 has developed a core dataset that can aid in gathering comparable data26, 27 and that is already used in many national research registries (https://treat-registry-taskforce.org/).
As a next step, it might be interesting to compare the results of this study with insurance company data. Also, as the treatment of AE will very likely change with the further introduction of new developments, it might be very interesting to re‐evaluate the prescribing practices in moderate‐to‐severe AE in the future and see how these changes have influenced patient care. This will be even more interesting in the future as our understanding of the molecular basis of AE advances and phenotypes and biomarkers that characterize these phenotypes are being unravelled.28
Supporting information
Table S1 Reasons for prescribing photo(chemo)therapy.
Table S2 Reasons against prescribing photo(chemo)therapy.
Table S3 Reasons for prescribing systemic therapy.
Table S4 Reasons against prescribing systemic therapy.
Table S5 Reasons for prescribing specific systemic therapies.
Table S6 Reasons against prescribing specific systemic therapies.
Table S7 National societies that received an invitation for the TREAT survey.
Table S8 Prescription patterns by workplace for (a) ciclosporin, (b) azathioprine, (c) methotrexate, (d) mycophenolic acid and (e) oral corticosteroids.
Table S9 First‐line systemic therapy per country.
Table S10 Second‐line systemic therapy per country.
Table S11 Third‐line systemic therapy per country.
Powerpoint S1 Journal Club Slide Set.
Acknowledgments
The authors would like to thank all of the participating (resident) dermatologists for their contributions to this TREAT survey.
Funding sources This study was supported through funding provided by the European Academy of Dermatology and Venereology (2016‐034).
Conflicts of interest A.D.I. has served as a consultant to AbbVie, Anacor, Chugai Pharma, Pfizer, Regeneron, Roche/Genentech, Sanofi‐Genzyme and UCB Pharma. C.F. is chief investigator of the UK–Irish Atopic Eczema Systemic Therapy Registry (A *STAR) and the UK National Institute for Health Research‐funded Treatment of Severe Atopic Eczema in Children Trial (TREAT). His department has also received funding from Sanofi for investigator‐led research. C.V. has advised and given lectures for AbbVie, LEO Pharma, Novartis and Sanofi‐Genzyme, and has been involved in the development of Patient‐Oriented Scoring Atopic Dermatitis. F.M.V. was involved as a subinvestigator in clinical trials for AbbVie, Novartis, LEO Pharma, Lilly and Regeneron. J.S. has received institutional funding for investigator‐initiated research from ALK, Novartis, Pfizer and Sanofi, and is chief investigator of the German AE registry TREATgermany. M.D. has been a speaker, advisory board member and/or investigator for AbbVie, CK‐Care Foundation, La Roche Posay Foundation, LEO Pharma, Meda Pharma, Pierre Fabre Laboratories, Regeneron and Sanofi‐Genzyme. P.I.S. has served as a consultant to AbbVie, Anacor, LEO Pharma, Novartis and Sanofi (unpaid); has received independent research grants from LEO Pharma and Schering‐Plough > 5 years ago; has been involved in performing clinical trials with many pharmaceutical companies that manufacture drugs used for the treatment of atopic eczema; and is chief investigator of the Dutch AE registry TREAT NL.
Plain language summary available online
References
- 1. Roekevisch E, Spuls PI, Kuester D et al Efficacy and safety of systemic treatments for moderate‐to‐severe atopic dermatitis: a systematic review. J Allergy Clin Immunol 2014; 133:429–38. [DOI] [PubMed] [Google Scholar]
- 2. Garritsen FM, Brouwer MW, Limpens J et al Photo(chemo)therapy in the management of atopic dermatitis: an updated systematic review with implications for practice and research. Br J Dermatol 2014; 170:501–13. [DOI] [PubMed] [Google Scholar]
- 3. Roekevisch E, Schram ME, Leeflang MMG et al Methotrexate versus azathioprine in patients with atopic dermatitis: 2‐year follow‐up data. J Allergy Clin Immunol 2018; 141:825–7. [DOI] [PubMed] [Google Scholar]
- 4. Gerbens LAA, Hamann SAS, Brouwer MWD et al Methotrexate and azathioprine for severe atopic dermatitis: a 5‐year follow‐up study of a randomized controlled trial. Br J Dermatol 2018; 178:1288–96. [DOI] [PubMed] [Google Scholar]
- 5. Nankervis H, Thomas KS, Delamere FM et al What is the evidence base for atopic eczema treatments? A summary of published randomized controlled trials. Br J Dermatol 2017; 176:910–27. [DOI] [PubMed] [Google Scholar]
- 6. European Medicines Agency . Dupixent. Summary of product characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/dupixent-epar-product-information_en.pdf (last accessed 26 February 2020).
- 7. Food and Drug Administration . DUPIXENT® (dupilumab) injection, for subcutaneous use. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761055lbl.pdf (last accessed 26 February 2020).
- 8. Alexander H, Patton T, Jabbar‐Lopez ZK et al Novel systemic therapies in atopic dermatitis: what do we need to fulfil the promise of a treatment revolution? F1000Res 2019; 8 (F1000 Faculty Rev):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Braddock M, Hanania NA, Sharafkhaneh A et al Potential risks related to modulating interleukin‐13 and interleukin‐4 signalling: a systematic review. Drug Saf 2018; 41:489–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sidbury R, Davis DM, Cohen DE et al Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol 2014; 71:327–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sidbury R, Kodama S. Atopic dermatitis guidelines: diagnosis, systemic therapy, and adjunctive care. Clin Dermatol 2018; 36:648–52. [DOI] [PubMed] [Google Scholar]
- 12. Proudfoot LE, Powell AM, Ayis S et al The European TREatment of severe Atopic eczema in children Taskforce (TREAT) survey. Br J Dermatol 2013; 169:901–9. [DOI] [PubMed] [Google Scholar]
- 13. Totri CR, Eichenfield LF, Logan K et al Prescribing practices for systemic agents in the treatment of severe pediatric atopic dermatitis in the US and Canada: the PeDRA TREAT survey. J Am Acad Dermatol 2017; 76:281–5. [DOI] [PubMed] [Google Scholar]
- 14. Taylor K, Swan DJ, Affleck A et al Treatment of moderate‐to‐severe atopic eczema in adults within the U.K.: results of a national survey of dermatologists. Br J Dermatol 2017; 176:1617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bruijnzeel‐Koomen CAFM, Spuls PI, Bruin‐Weller MS et al NVDV Richtlijn: constitutioneel eczeem [Directive: constitutional eczema]. Available at: https://mijn.venvn.nl/databanken/richtlijnen/Lists/Databank%20richtlijnen/Attachments/105/Richtlijn%20Constitutioneel%20Eczeem%20update%202014.pdf (last accessed 26 February 2020).
- 16. Wollenberg A, Barbarot S, Bieber T et al Consensus‐based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol 2018; 32:657–82. [DOI] [PubMed] [Google Scholar]
- 17. Wollenberg A, Barbarot S, Bieber T et al Consensus‐based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol 2018; 32:850–78. [DOI] [PubMed] [Google Scholar]
- 18. LePoidevin LM, Lee DE, Shi VY. A comparison of international management guidelines for atopic dermatitis. Pediatr Dermatol 2019; 36:36–65. [DOI] [PubMed] [Google Scholar]
- 19. Ling TC, Clayton TH, Crawley J et al British Association of Dermatologists and British Photodermatology Group guidelines for the safe and effective use of psoralen‐ultraviolet A therapy 2015. Br J Dermatol 2016; 174:24–55. [DOI] [PubMed] [Google Scholar]
- 20. European Medicines Agency . Sandimmun Neoral and associated names. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Sandimmun_Neoral_and_associated_names/human_referral_000344.jsp&mid=WC0b01ac05805c516f (last accessed 26 February 2020).
- 21. van der Schaft J, Politiek K, van den Reek J et al Drug survival for ciclosporin A in a long‐term daily practice cohort of adult patients with atopic dermatitis. Br J Dermatol 2015; 172:1621–7. [DOI] [PubMed] [Google Scholar]
- 22. Vestergaard C, Wollenberg A, Barbarot S et al European task force on atopic dermatitis position paper: treatment of parental atopic dermatitis during preconception, pregnancy and lactation period. J Eur Acad Dermatol Venereol 2019; 33:1644–59. [DOI] [PubMed] [Google Scholar]
- 23. Drucker AM, Eyerich K, de Bruin‐Weller MS et al Use of systemic corticosteroids for atopic dermatitis: International Eczema Council consensus statement. Br J Dermatol 2018; 178:768–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Drucker AM, Ellis A, Jabbar‐Lopez Z et al Systemic immunomodulatory treatments for atopic dermatitis: protocol for a systematic review with network meta‐analysis. BMJ Open 2018; 8:e023061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spuls PI, Gerbens LAA, Apfelbacher CJ et al The International TREatment of ATopic Eczema (TREAT) Registry Taskforce: an initiative to harmonize data collection across national atopic eczema photo‐ and systemic therapy registries. J Invest Dermatol 2017; 137:2014–16. [DOI] [PubMed] [Google Scholar]
- 26. Gerbens LAA, Apfelbacher CJ, Irvine AD et al TREatment of ATopic eczema (TREAT) Registry Taskforce: an international Delphi exercise to identify a core set of domains and domain items for national atopic eczema photo‐ and systemic therapy registries. Br J Dermatol 2019; 180:790–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vermeulen FM, Gerbens LAA, Bosma AL et al TREatment of ATopic eczema (TREAT) Registry Taskforce: consensus on how and when to measure the core dataset for atopic eczema treatment research registries. Br J Dermatol 2019; 181:492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cabanillas B, Brehler AC, Novak N. Atopic dermatitis phenotypes and the need for personalized medicine. Curr Opin Allergy Clin Immunol 2017; 17:309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Reasons for prescribing photo(chemo)therapy.
Table S2 Reasons against prescribing photo(chemo)therapy.
Table S3 Reasons for prescribing systemic therapy.
Table S4 Reasons against prescribing systemic therapy.
Table S5 Reasons for prescribing specific systemic therapies.
Table S6 Reasons against prescribing specific systemic therapies.
Table S7 National societies that received an invitation for the TREAT survey.
Table S8 Prescription patterns by workplace for (a) ciclosporin, (b) azathioprine, (c) methotrexate, (d) mycophenolic acid and (e) oral corticosteroids.
Table S9 First‐line systemic therapy per country.
Table S10 Second‐line systemic therapy per country.
Table S11 Third‐line systemic therapy per country.
Powerpoint S1 Journal Club Slide Set.


