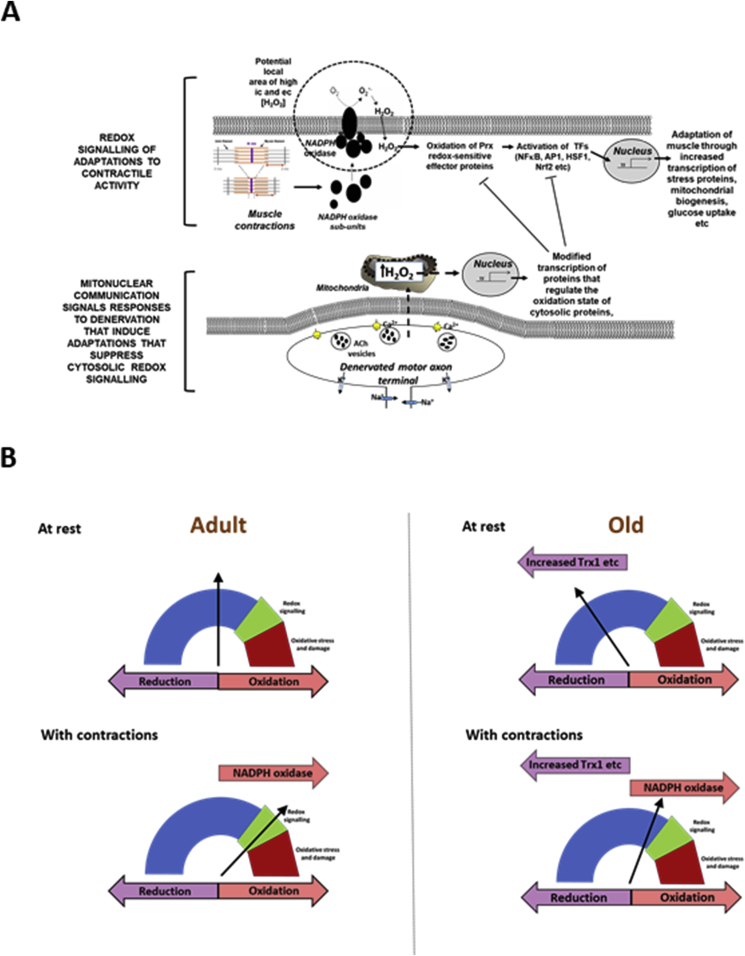
Fig. 7.
A. Schematic representation of how peroxides generated in muscle mitochondria in response to denervation may suppress redox signalling of adaptations to contractions. It was originally envisaged that peroxides generated in mitochondria as a result of denervation might directly act on NADPH oxidase mediated redox signalling pathways, but compartmentalisation prevents such direct interference. It is proposed that the increased mitochondrial stress triggers reverse mitonuclear communication that leads to upregulation of regulatory pathways, such as increased Trx, GPx1, catalase [60] and Prx [63], that causes an increased reductive environment and suppression of redox-sensitive contraction-induced adaptations.
B. Hypothetical representation of the redox status of critical cysteines involved in signalling responses to contractions in proteins such as the “peroxidatic” cysteine in Prx2. In adult mice at rest the redox balance reflects baseline generation of H2O2 and other oxidising agents and the local amount and activity of reductants and regulatory proteins. During exercise activation of NADPH oxidase leads to a shift to a more oxidised status which is sufficient to lead to oxidation of further signalling proteins potentially through a redox relay system. In old mice at rest the redox status of the critical cysteine is shifted to a more reduced state due to the adaptive upregulation of proteins, such as Trx1 which reduce the critical cysteine. During exercise in old mice the oxidation stimulus achieved by activation of NADPH oxidase is therefore insufficient to modify the cysteine sufficiently to activate the signalling pathways.