Box 1, Figure I.
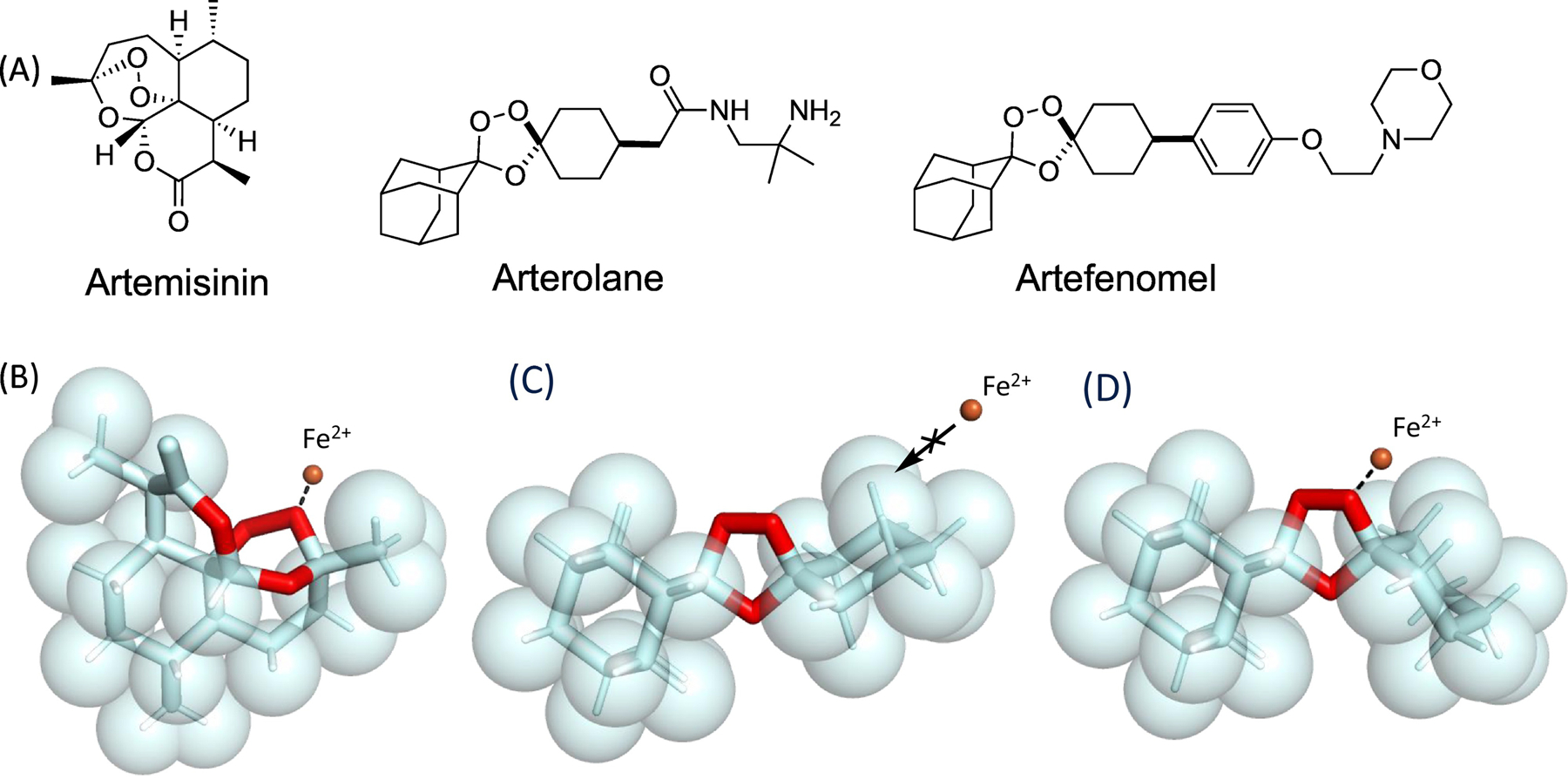
Structure-reactivity relationships of antimalarial peroxides. A. Structures of antimalarials artemisinin, arterolane, and artefenomel. B. Spacefilling representation of artemisinin showing approach (dashed line) of Fe2+ ion (orange sphere) to the more exposed of two peroxidic oxygen atoms. C. Spacefilling representation of arterolane pharmacophore in its major, unreactive conformer, with approach of the Fe2+ ion sterically blocked by proximal axial C–H bonds of the adamantane and cyclohexane rings. D. Spacefilling representation of the corresponding iron-reactive, peroxide equatorial conformer showing approach of the Fe2+ ion (orange sphere) to the exposed peroxidic oxygen atom. Artemisinin structure retrieved from the Cambridge Crystallographic Data Centrei and visualized using PyMol 2.4.0 (pymol.org). Arterolane pharmacophore structure minimized using MarvinSketch 6.3.0 (chemaxon.com) and visualized using PyMol 2.4.0 (pymol.org).
