Figure 1.
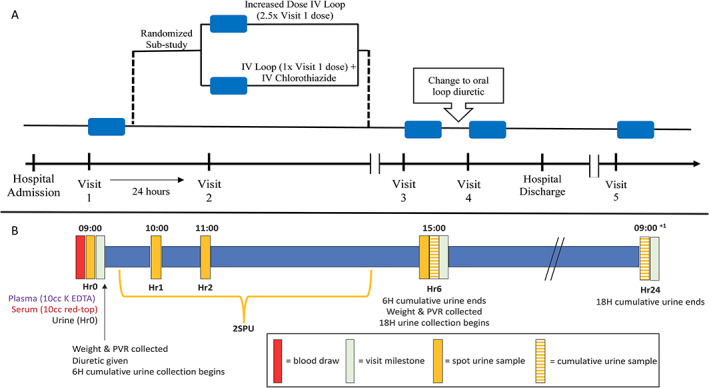
Study assessment diagram. (A) Patients hospitalized with acute decompensated heart failure enrolled in the Mechanisms of Diuretic Resistance Study undergo serial ‘visits’, indicated by a blue box. Visits 1, 3, and 4 occur during open‐label intravenous (IV) loop diuretic therapy. Patients who are diuretic resistant at Visit 1 and meet sub‐study criteria are randomized to increased loop diuretic therapy or combination of loop and thiazide diuretic therapy at Visit 2 the following day. Visit 3 occurs during IV diuretic therapy later in the hospital stay. Visit 4 occurs on the day of oral diuretic therapy prior to hospital discharge. Visit 5 occurs in a clinical research centre after hospital discharge on oral diuretic therapy. (B) Each visit includes a supervised 6 h urine collection with urine and blood sample collection and standing weight measurements timed to a dose of loop diuretic. Spot urine is collected at Hours 0, 1, 2, and 6. A spot sample from the second spontaneous urination (2SPU) is also collected if it occurred outside of these timed spot urine samples. A sample from the 6 and 18 h cumulative urine collections is also collected. H, cumulative hours; Hr, hour of biospecimen collection; PVR, post‐void residual.
