Abstract
Aims
Increasing attention is being given to patients with heart failure and ‘mid‐range’ left ventricular ejection fraction (LVEF, ≥40% and <50%) for whom there are no approved therapies that improve prognosis. We aim to assess for the first time the effects of cardiac contractility modulation (CCM) therapy in this patient population.
Methods and results
We assessed the effects of 6‐ month CCM therapy on functional status, exercise tolerance and quality of life in a subgroup of 53 patients with a LVEF of 40–45% recruited in previous CCM studies, including 37 patients in the CCM group and 16 in the control group. New York Heart Association classification improved by ≥1 class from baseline to 24 weeks in 80.6% (95% confidence interval [62.5%, 92.5%]) of patients in the CCM group compared with 57.1% in the control group (95% confidence interval [28.9%, 82.3%], P = 0.15). Six‐minute walk distance increased significantly in the CCM group with a net between‐group treatment effect of 53.9 ± 74.2 m (P = 0.05). Peak VO2 improved in the CCM group with a net between‐group treatment effect of 2.0 ± 2.8 mL/kg/min (P = 0.02). Minnesota Living with Heart Failure Questionnaire score decreased from baseline to 24 weeks with a net between‐group treatment effect of −13.1 ± 21.0 (P = 0.10). There were no significant differences in the adverse event rate between the CCM and control groups.
Conclusions
These preliminary results suggest that CCM exerts favourable effects on exercise tolerance and quality of life in patients with LVEF in the range of 40–45% with an acceptable safety profile. Further randomized controlled studies are planned to prove these effects.
Keywords: Heart failure, Mid‐range ejection fraction, Mildly reduced ejection fraction, Cardiac contractility modulation, Quality of life
Introduction
Cardiac contractility modulation (CCM) has been shown to improve quality of life, functional status, and exercise tolerance in patients with New York Heart Association (NYHA) Class III symptoms, despite guideline‐directed medical therapies (GDMTs) and left ventricular (LV) ejection fraction (LVEF) between 25% and 45%. 1 , 2 , 3 , 4 While data have shown that benefits are appreciated across this entire spectrum, patients with a LVEF of 35–45% showed stronger efficacy signals than those with a LVEF of 25–35%. 5 , 6 In the prior subgroup analyses, however, a majority of patients in the higher LVEF group actually fell between 35% and 40%, so that there is minimal information regarding CCM efficacy in patients with LVEF > 40%. Recently, increased attention has been devoted to describing the characteristics and outcomes in patients with heart failure in the setting of mildly reduced LVEF (40% < LVEF < 50%) for which there are no approved therapies. At the same time, additional data concerning CCM effects in patients with LVEF ≥ 40% have become available. 1 , 2
In this subgroup analysis, the effects of 6 months CCM treatment plus GDMT on quality of life, exercise tolerance, and functional status were assessed and compared with a control group receiving GDMT alone; both treatment and control patients were enrolled in prior studies of CCM that used similar inclusion and exclusion criteria and follow‐up schedules (Supporting Information, Table S1 ).
Patients and methods
In this subgroup analysis of efficacy and safety, data were obtained from patients with baseline LVEF ≥ 40% from the FIX‐HF‐5, FIX‐HF‐5C, and FIX‐HF‐5C2 studies. 1 , 2 The primary efficacy analyses in all three studies were performed at 6 months following study inclusion. The Optimizer IV device was used in the FIX‐HF‐5 and FIX‐HF‐5C studies, while the Optimizer Smart device was used in the FIX‐HF‐5C2 study. The characteristics of the CCM therapy delivered by these two devices are identical. The major differences between the devices are that Optimizer IV requires an atrial and two right ventricular (RV) leads, whereas Optimizer Smart requires only two RV leads. In all, there were 53 patients with LVEF ≥ 40%, 37 in a treatment group and 16 in a control group. In all of these patients, LVEF ranged from 40% to 45%.
Effectiveness measures
The key measure of efficacy was peak oxygen consumption (pVO2) during standardized cardiopulmonary stress test that was evaluated at a blinded core laboratory; pVO2 was measured in all three studies. NYHA functional class was also assessed in all three studies. Six minute walk distance and quality of life assessed by the Minnesota Living with Heart Failure Questionnaire (MLWHFQ) were measured in the FIX‐HF‐5 and FIX‐HF‐5C studies only.
Safety measures
In all studies, serious adverse events, hospitalizations, and mortality were adjudicated by an independent Events Adjudication Committee as detailed previously. 1 , 2 , 3
Statistical analyses
Results are expressed as changes from baseline, and comparisons were made between patients who received 6 months of CCM treatment with continued GDMT (treatment group) and those receiving only GDMT (control group). Baseline characteristics were compared between groups via Fisher's exact test for binary variables and two‐sample t‐tests for continuous variables. Between‐group differences in changes of effectiveness parameters from baseline, except for NYHA class, were compared by paired t‐tests and analysis of covariance adjusted for baseline values. Between‐group differences in changes of NYHA class were compared with Fisher's exact test and logistic regression adjusted for baseline value. Between‐group comparisons in the incidences of serious adverse events were performed via Fisher's exact test. Time‐to‐first hospitalizations and deaths were compared between the groups by Kaplan–Meier survival analysis.
Results
Baseline characteristics
There were no statistically significant differences between the treatment and control groups with respect to baseline characteristics (Table 1 ).
Table 1.
Baseline characteristics
| Variable | CCM Optimizer EF ≥ 40% | Control EF ≥ 40% | P‐value |
|---|---|---|---|
| N = 37 | N = 16 | ||
| Demographics | |||
| Age (years) | 62.4 ± 12.1 | 65.0 ± 10.4 | 0.46 |
| Male | 26 (70.3%) | 13 (81.3%) | 0.51 |
| BMI (kg/m2) | 32.4 ± 7.4 | 31.9 ± 7.4 | 0.83 |
| History | |||
| Ischaemic aetiology | 19 (51.4%) | 8 (50.0%) | 1.00 |
| Prior ICD or PM system | 29 (85.3%) | 14 (87.5%) | 1.00 |
| Diabetes mellitus | 17 (45.9%) | 8 (50.0%) | 1.00 |
| Atrial fibrillation | 16 (43.2%) | 3 (18.8%) | 0.12 |
| Ventricular arrhythmias | 7 (18.9%) | 7 (43.8%) | 0.09 |
| Baseline evaluation | |||
| NYHA Class III | 33 (89.2%) | 13 (81.3%) | 0.42 |
| NYHA Class IV | 4 (10.8%) | 3 (18.8%) | 0.42 |
| Resting HR (b.p.m.) | 74.7 ± 10.8 | 68.5 ± 11.8 | 0.069 |
| Systolic BP (mmHg) | 121.4 ± 15.0 | 126.3 ± 20.0 | 0.34 |
| Diastolic BP (mmHg) | 74.0 ± 9.5 | 72.9 ± 11.3 | 0.71 |
| QRS duration (ms) | 98.1 ± 13.1 | 102.6 ± 16.6 | 0.30 |
| LVEF (%) (core lab) | 42.1 ± 1.7 | 41.8 ± 1.7 | 0.62 |
| LVEDD (mm) (core lab) | 52.6 ± 6.5 | 54.9 ± 5.3 | 0.21 |
| Peak VO2 (mL/kg/min) | 15.5 ± 3.1 | 16.1 ± 2.4 | 0.51 |
| Medications | |||
| ACEi/ARB/ARNi | 21 (56.8%) | 7 (43.8%) | 0.55 |
| Beta‐blocker | 28 (75.7%) | 10 (62.5%) | 0.34 |
| MRA | 10 (27.0%) | 5 (31.3%) | 0.75 |
| Diuretic | 23 (62.2%) | 9 (56.3%) | 0.76 |
| Digoxin | 3 (8.1%) | 0 (0.0%) | 0.55 |
| Anti‐arrhythmic | 9 (24.3%) | 2 (12.5%) | 0.47 |
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor neprilysin inhibitor; BMI, body mass index; BP, blood pressure; CCM, cardiac contractility modulation; EF, ejection fraction; HR, heart rate; ICD, implantable cardio‐defibrillator; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; PM, pacemaker; V02, oxygen uptake.
Effectiveness
A comparison of observed changes in efficacy measures in CCM and control groups is summarized in Figure 1 . NYHA classification improved by ≥1 class from baseline to 24 weeks in 80.6% (95% confidence interval [62.5%, 92.5%]) of patients in the CCM group compared with 57.1% in the control group (95% confidence interval [28.9%, 82.3%], P = 0.15). Six‐minute walk distance increased significantly in the CCM group by 70.5 ± 51.7 m from baseline to 24 weeks (P = 0.001) but did not change significantly in the control group (16.6 ± 94.9 m), leading to an average between‐group treatment effect of 53.9 ± 74.2 m (P = 0.05). Peak VO2 improved in the CCM group by 0.9 ± 3.0 mL/kg/min and declined by 1.2 ± 2.0 mL/kg/min in the control group for a net between‐group treatment effect of 2.0 ± 2.8 mL/kg/min (P = 0.02). Finally, MLWHFQ decreased from baseline to 24 weeks by 18.9 ± 22.4 in the CCM group compared with a decrease of 5.9 ± 19.3 in the control group, for a between‐group treatment effect of −13.1 ± 21.0 (P = 0.10).
Figure 1.
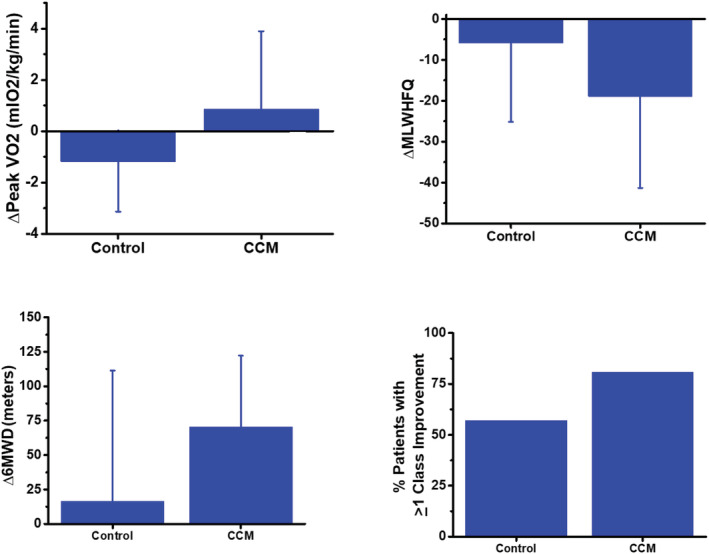
Absolute changes in exercise capacity, quality of life, and functional status observed in the cardiac contractility modulation (CCM) group and the control group (6MWD, 6 min walk distance; MLWHFQ, Minnesota Living with Heart Failure Questionnaire).
Safety adverse events
Adverse event profiles were typical of the patient population studied (Supporting Information, Table S2 ), and there were no significant differences between the groups for any adverse event captured.
Mortality and hospitalizations
Kaplan–Meier estimates of freedom from cardiovascular mortality (97.2% vs. 100%; P = 0.51) and freedom from the composite of heart failure hospitalization or cardiovascular mortality (91.7% vs. 93.8%; P = 0.79) did not differ between groups.
Discussion
While CCM was initially studied in patients with reduced LVEF, 3 , 4 prior subgroup analyses have suggested that efficacy effects are greater in patients on the higher end of the LVEF range. 5 , 6 This was specifically shown in a prior subgroup analysis that primarily included patients with LVEF between 35% and 40%. 5 By including a small number of patients pooled from three prior studies with similar inclusion criteria, assessments and follow‐up, the current results suggest that compared to a group of similarly selected control patients, CCM also exerts favourable effects on exercise tolerance and quality of life in patients with LVEF between 40% and 45%.
A recently performed individual‐patient meta‐analysis of 861 patients from randomized trials of CCM therapy 7 also demonstrated improvements in peak VO2, 6‐ min walk distance, and quality of life, thus confirming the findings of the present study. Moreover, long‐term improvements in quality of life, as estimated by NYHA class and MLWHFQ, along with reductions in rates of hospitalization were seen in another analysis 8 of 140 patients with LVEF of 25–45% included in the European Union Registry of CCM therapy.
The mechanism of improved outcomes with CCM therapy in patients with higher LVEF has been hypothesized to be multifactorial. 9 Studies of myocardium from preclinical models of heart failure and from human biopsy specimens have shown that after 3 months of CCM therapy, calcium uptake, phosphorylation of myofilaments, and phosphorylation of titin (which enhances titin distensibility) are all enhanced. 9 , 10 , 11 Reduction in oxidative stress, cardiac fibrosis, and sympathetic activity have also been associated with CCM therapy. 9 This was further found in endomyocardial biopsies from the RV and LV 3 months after CCM in a small case report series of patients with heart failure with preserved ejection fraction. Interestingly, these changes were found both in the area of CCM stimulation in RV, as well in remote areas in LV, suggesting both local and general effects 12 (Supporting Information, Table S3 ).
Collectively, these actions may contribute to the beneficial physiological effects of CCM on the hearts of patients with higher LVEFs where, in addition to reduced myocardial contractility, increased myocardial stiffness is believed to contribute to heart failure symptoms. Assuredly, other explanations for the clinical effects of CCM therapy in patients with higher ejection fractions remain to be elucidated.
Nevertheless, these basic and clinical observations, coupled with preliminary experience in a small number of patients with LVEF > 50%, 9 have prompted initiation of a European pilot study of patients with LVEF ranging from 40% to 60%, which is currently enrolling (ClinicalTrials.gov identifier: NCT03240237). Furthermore, a multinational, pivotal, randomized, double‐blind study is currently being planned. It is only through such studies that definitive proof of safety and efficacy of CCM in heart failure patients with higher LVEF can be obtained in order to determine the upper bound of LVEF where CCM clinical benefits extend.
Conclusions
These preliminary results derived by a subgroup analysis suggest that CCM therapy exerts favourable effects on functional status, exercise tolerance, and quality of life in patients with LVEF in the range of 40–45%, with an acceptable safety profile. Further randomized controlled studies are planned to prove these effects.
Conflict of interest
C.T. has received speaker fees and/or contributions to congresses from Abbott, Abiomed, AstraZeneca, Bayer, Impulse Dynamics, Novartis, Pfizer, and Servier. J.B. is a consultant to Abbott, Amgen, Array, AstraZeneca, Bayer, Boehringer Ingelheim, CVRx, Eli Lilly, G3 Pharmaceuticals, Impulse Dynamics, Innolife, Janssen, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Sequana, Stealth Peptides, and Vifor. D.F. has received consultation fees and/or speaker honoraria from Abbott Laboratories, Bayer, Boehringer Ingelheim, Leo, Menarini, Novartis, Orion Pharma, and Roche Diagnostics. D.M. and I.R. are employees of Impulse Dynamics. G.F. has served as member of committees in trials sponsored by Amgen, Bayer, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor.
Supporting information
Table S1: Key Inclusion and Exclusion Criteria
Table S2: Serious Adverse Events
Table S3: Proposed mechanisms of CCM in heart failure across the entire range of ejection fraction
Tschöpe, C. , Butler, J. , Farmakis, D. , Morley, D. , Rao, I. , and Filippatos, G. (2020) Clinical effects of cardiac contractility modulation in heart failure with mildly reduced systolic function. ESC Heart Failure, 7: 3531–3535. 10.1002/ehf2.13126.
References
- 1. Wiegn P, Chan R, Jost C, Saville BR, Parise H, Prutchi D, Carson PE, Stagg A, Goldsmith RL, Burkhoff D. Safety, performance, and efficacy of cardiac contractility modulation delivered by the 2‐lead Optimizer Smart System: the FIX‐HF‐5C2 study. Circ Heart Fail 2020; 13: e006512. [DOI] [PubMed] [Google Scholar]
- 2. Abraham WT, Kuck KH, Goldsmith RL, Lindenfeld J, Reddy VY, Carson PE, Mann DL, Saville B, Parise H, Chan R, Wiegn P, Hastings JL, Kaplan AJ, Edelmann F, Luthje L, Kahwash R, Tomassoni GF, Gutterman DD, Stagg A, Burkhoff D, Hasenfuss GA. Randomized controlled trial to evaluate the safety and efficacy of cardiac contractility modulation. JACC Heart Fail 2018; 6: 874–883. [DOI] [PubMed] [Google Scholar]
- 3. Kadish A, Nademanee K, Volosin K, Krueger S, Neelagaru S, Raval N, Obel O, Weiner S, Wish M, Carson P, Ellenbogen K, Bourge R, Parides M, Chiacchierini RP, Goldsmith R, Goldstein S, Mika Y, Burkhoff D, Abraham WT. A randomized controlled trial evaluating the safety and efficacy of cardiac contractility modulation in advanced heart failure. Am Heart J 2011; 161: 329, e1–2–337. [DOI] [PubMed] [Google Scholar]
- 4. Borggrefe MM, Lawo T, Butter C, Schmidinger H, Lunati M, Pieske B, Misier AR, Curnis A, Bocker D, Remppis A, Kautzner J, Stuhlinger M, Leclerq C, Taborsky M, Frigerio M, Parides M, Burkhoff D, Hindricks G. Randomized, double blind study of non‐excitatory, cardiac contractility modulation electrical impulses for symptomatic heart failure. Eur Heart J 2008; 29: 1019–1028. [DOI] [PubMed] [Google Scholar]
- 5. Borggrefe M, Burkhoff D. Clinical effects of cardiac contractility modulation (CCM) as a treatment for chronic heart failure. Eur J Heart Fail 2012; 14: 703–712. [DOI] [PubMed] [Google Scholar]
- 6. Abraham WT, Nademanee K, Volosin K, Krueger S, Neelagaru S, Raval N, Obel O, Weiner S, Wish M, Carson P, Ellenbogen K, Bourge R, Parides M, Chiacchierini RP, Goldsmith R, Goldstein S, Mika Y, Burkhoff D, Kadish A, FIX‐HF‐5 Investigators and Coordinators . Subgroup analysis of a randomized controlled trial evaluating the safety and efficacy of cardiaccontractility modulation in advanced heart failure. J Card Fail 2011; 17: 710–717. [DOI] [PubMed] [Google Scholar]
- 7. Giallauria F, Cuomo G, Parlato A, Raval N, Kuschyk J, Coats A. A comprehensive individual patient data meta‐analysis of the effects of cardiac contractility modulation on functional capacity and heart failure related quality of life. ESC Heart Fail 2020; 7: 2922–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anker S, Borggrefe M, Neuser H, Ohlow MA, Röger S, Goette A, Remppis BA, Kuck KH, Najarian KB, Gutterman DD, Rousso B. Cardiac contractility modulation improves long term survival and hospitalizations in heart failure with reduced ejection fraction. Eur Heart Fail J 2019; 21: 1103–1113. [DOI] [PubMed] [Google Scholar]
- 9. Tschope C, Kherad B, Klein O, Lipp A, Blaschke F, Gutterman D, Burkhoff D, Hamdani N, Spillmann F, Van Linthout S. Cardiac contractility modulation: mechanisms of action in heart failure with reduced ejection fraction and beyond. Eur J Heart Fail 2019; 21: 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Imai M, Rastogi S, Gupta RC, Mishra S, Sharov VG, Stanley WC, Mika Y, Rousso B, Burkhoff D, Ben‐Haim S, Sabbah HN. Therapy with cardiac contractility modulation electrical signals improves left ventricular function and remodeling in dogs with chronic heart failure. J Am Coll Cardiol 2007; 49: 2120–2128. [DOI] [PubMed] [Google Scholar]
- 11. Butter C, Rastogi S, Minden HH, Meyhofer J, Burkhoff D, Sabbah HN. Cardiac contractility modulation electrical signals improve myocardial gene expression in patients with heart failure. J Am Coll Cardiol 2008; 51: 1784–1789. [DOI] [PubMed] [Google Scholar]
- 12. Tschöpe C, Van Linthout S, Spillmann F, Klein O, Biewener S, Remppis A, Gutterman D, Linke WA, Pieske B, Hamdani N, Roser M. Cardiac contractility modulation signals improve exercise intolerance and maladaptive regulation of cardiac key proteins for systolic and diastolic function. Int J Cardiol 2016; 203: 1061–1066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Key Inclusion and Exclusion Criteria
Table S2: Serious Adverse Events
Table S3: Proposed mechanisms of CCM in heart failure across the entire range of ejection fraction


