Abstract
Alzheimer’s disease is a neuropathological condition with abnormal accumulation of extracellular Amyloid-β plaques and intracellular neurofibrillary tangles of Microtubule-associated protein Tau (Tau) in the brain. In pathological conditions, Tau undergoes post-translational modifications such as hyperphosphorylation by the activity of cellular kinases, which eventually leads to protein aggregation in neurons. Melatonin is a neuro-hormone that is mainly secreted from the pineal gland and functions to modulate the cellular kinases. In our study, we have checked the neuroprotective function of Melatonin by MTT and LDH assay, where Melatonin inhibited the Tau aggregates-mediated cytotoxicity and membrane leakage in Neuro2A cells. The potency of Melatonin has also been studied for the quenching of intracellular reactive oxygen species level by DCFDA assay and caspase 3 activity. Melatonin was shown to reduce the GSK3β mRNA and subsequent protein level as well as the phospho-Tau level (pThr181 and pThr212-pSer214) in okadaic acid-induced Neuro2A cells, as observed by western blot and immunofluorescence assay. Further, Melatonin has increased the cellular Nrf2 level and its nuclear translocation as an oxidative stress response in Tauopathy. The Melatonin was found to induce pro- and anti-inflammatory cytokines levels in N9 microglia. The mRNA level of cellular kinases such as as-GSK3β, MAPK were also studied by qRT-PCR assay in Tau-exposed N9 and Neuro2A cells. The immunomodulatory effect of Melatonin was evident as it induced IL-10 and TGF-β cytokine levels and activated MAP3K level in Tau-exposed microglia and neurons, respectively. Melatonin also downregulated the mRNA level of pro-inflammatory markers, IL-1β and Cyclooxygenase-2 in N9 microglia. Together, these findings suggest that Melatonin remediated the cytokine profile of Tau-exposed microglia, reduced Tau hyperphosphorylation by downregulating GSK3β level, and alleviated oxidative stress via Nrf2 nuclear translocation.
Keywords: melatonin, neurodegeneration, GSK3β, Nrf2, microglia, Tau hyperphosphorylation, anti-inflammatory, Alzheimer’s disease
Introduction
Alzheimer’s disease (AD) is a chronic neurodegenerative disease that is associated with extracellular β-amyloid plaques and intracellular deposition of neurofibrillary tangles (NFTs) of Tau in the central nervous system (CNS). Tau is a natively unfolded cytosolic protein that helps in microtubule stabilization and axonal transport (Johnson and Hartigan, 1999). Tau contains a variety of post-translational modifications (PTMs), of which phosphorylation is the most common in AD. Tau can be phosphorylated by various cellular kinases such as as-GSK3β, CDK5-P25, PKA, etc. The phosphorylated residues of Tau, such as- pThr231 (PHF6), pSer202-pThr205 (AT8), pSer212-pThr214 (AT100), pThr181 (AT270) are most common epitopes in Tauopathy (Mandelkow and Mandelkow, 2012). The hyperphosphorylation of Tau results in the insult of its microtubule-binding function, which subsequently leads to its self-association and accumulation in the neurons as NFTs (Matsuo et al., 1994). Cellular proteostasis machinery regulates the clearance of aggregated Tau either by proteasomal pathway or by chaperone-mediated autophagy, which directs towards lysosomal degradation as unfolded protein response (Gorantla and Chinnathambi, 2018). Pathological Tau species can be secreted from neurons via exosomes, along with neurotransmitters or through diffusion into synapses (Guo and Lee, 2014). The hyperphosphorylated Tau and extracellular multimeric species can propagate through neuronal synapses and act as seed species for the aggregation of intracellular Tau in healthy neurons (Sonawane and Chinnathambi, 2018). The Tau NFTs containing neurons flag phosphatidylserine on its surface and activates the signaling cascade for apoptosis (Brunello et al., 2020). Microglia are residential macrophages in the brain, which primarily serve the function of immune surveillance, phagocytosis of evading microorganisms, cellular debris, plaques and help in the maintenance of synaptic plasticity (Tremblay et al., 2011). Upon encountering aggregates and damaged neurons, microglia become activated, resulting in increased phagocytic activity and releasing cytokines (Bedard and Krause, 2007; Schetters et al., 2018). Interleukin 1 (IL 1) secreted by microglia induces the stripping of myelin from the neuronal axons and attacks the affected neurons via NMDA receptor-function. (Gehrmann et al., 1995, Sierra et al., 2013). Activated microglia also remodel membrane-associated actin network for active migration and effective phagocytosis of extracellular Tau oligomers (Das et al., 2020). But the overactivation of microglia can lead to the engulfment of neuronal synapses, which results in memory loss and neurodegeneration in AD (Hong et al., 2016).
Melatonin is N-acetyl 5-methoxy tryptamine, a small hormone mainly produced from the pineal gland mainly involved in the regulation of circadian rhythm (Claustrat et al., 2005). It functions as a free radical scavenger, anti-apoptotic agent, anti-cancer, neuroprotectant and immune modulator. (Balmik and Chinnathambi, 2018). Melatonin is a known antioxidant molecule, which not only scavenges reactive oxygen species (ROS) but also modulates the activity of antioxidant enzymes like glutathione peroxidase, glutathione reductase, superoxide dismutase etc. (Zhang and Zhang, 2014). Melatonin plays a crucial role in mitochondrial homeostasis and stabilizes electron transport chain, ATP production and mitochondrial transportation (David et al., 2005, López et al., 2009). It has been reported that age-related extracellular patho-protein burden induces the oxidative stress response and caspase activation along with the inflammatory burst by activated astrocytes and microglia (Means et al., 2020). Melatonin treatment has been shown to improve cognitive function (Lin et al., 2013), lessen aggregate burden and sleeplessness in neurodegenerative diseases (Quinn et al., 2005). Melatonin can also induce the expression of nuclear factor erythroid-2 related factor (Nrf2) to reduce the oxidative stress in rat urinary bladder cells. Nrf2 is an essential transcription factor, which induces the expression of anti-oxidant mediators (Tripathi and Jena, 2010). Melatonin stalls the aggregation of β-amyloid peptide and inhibits the production of pro-inflammatory cytokines (IL 1, IL 6, TNF-α [tumor necrosis factor α]) in β-amyloid rat model (Rosales-Corral et al., 2003). Melatonin interferes with the inflammatory process by interacting with transcription factor NFκB and reduces the iNOS activity in J774 and RAW 264.7 murine macrophages (Gilad et al., 1998). Melatonin is also involved in the signaling cross-talk between Nrf2 and NFκB to modulate the neuroinflammation and mediate anti-oxidative stress response in the experimental neurodegenerative model (Negi et al., 2011). Additionally, Melatonin is involved in minimizing the production of endothelial adhering molecules, including Interstitial cell adhesion molecules (ICAM), vesicular adhering molecule (VCAM-I), endothelial selectin (E-selectin), related to reduced inflammatory burst (Motilva et al., 2011). Melatonin ameliorates the amyloid-β pathology via PI3-Akt signaling and ultimately reduces the Tau hyperphosphorylation in the mouse hippocampus (Ali and Kim, 2005). Hence, Melatonin can be considered as a promising therapeutic aid for its multi-faceted function in the scenario of neurodegeneration (Balmik and Chinnathambi, 2018).
In this study, we investigated the neuroprotective role of Melatonin in Tau-mediated cytotoxicity and its potential in reducing the oxidative stress and apoptotic loss in Tauopathies. We identified the efficacy of Melatonin in the reduction of Tau phosphorylation, GSK3β expression and Nrf2 translocation under oxidative stress response and the alteration of the cytokine profile of microglial cell line (N9) during Tau-exposed inflammation.
Materials and Methods
Cell Cultures and Reagents
Ampicillin, NaCl, Phenylmethylsulfonylfluoride (PMSF), MgCl2, APS, DMSO, Methanol, Ethanol, Chloroform, Isopropanol were purchased from MP Biomedicals; IPTG and Dithiothreitol (DTT) from Calbiochem; MES, BES, SDS, 2″-7″-Dichlorodihydrofluorescein diacetate (DCFDA), Okadaic acid, TritonX-100 from Sigma; EGTA, Protease inhibitor cocktail (PIC), Tris base, 40% Acrylamide, TEMED, Horse serum, Goat anti-Rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody HRP (A16110), DAPI from Invitrogen.
Preparation of Tau Aggregates and Transmission Electron Microscopy
The Tau protein purification was carried out as described previously (Gorantla et al., 2017). In brief, the full-length human pT7C Tau (4R2N) was expressed in E.coli BL21* in Luria-Bertani broth (Himedia) containing 100 μg/ml of ampicillin and induced with 0.5 mM IPTG for 3 hours at 37°C. The bacteria were harvested and lysed by Constant cell disruption system (Constant Systems Ltd.) at 15 kpsi pressure. The lysate was heated at 90°C and centrifuged at 45,000 rpm for 45 minutes. The supernatant was thoroughly dialyzed against 20 mM MES pH 6.8, 50 mM NaCl at 4°C overnight and further centrifuged at 40,000 rpm for 45 minutes. The protein was purified by cation-exchange chromatography with 20 mM MES pH 6.8, 50 mM NaCl for column wash and 20 mM MES pH 6.8, 1 M NaCl for elution of Tau protein. Final purification was done by size-exclusion chromatography in 1X PBS buffer, 2 mM DTT using Superdex 75 Hi-load 16/600 column. The concentration of Tau protein was measured by the BCA assay. Tau aggregates were prepared by inducing with Heparin (17.5 kDa) in 20 mM BES buffer containing DTT, NaCl, PIC, pH 7.4 (Heparin: Tau Molar ratio-1:4) and incubated for 7 days at 37°C (Barghorn et al., 2005). The formation of mature fibrils was checked by pelleting assay, where the mature fibril containing reaction mixture was centrifuged to 60,000 rpm for 60 minutes. The total, supernatant and pellet fractions were subjected to SDS-PAGE for the detection of matured Tau fibrils. The morphology of Tau fibrils was observed by using Transmission electron microscopy (TEM). The Tau aggregates were spotted onto carbon mesh containing copper grid (Ted Pella Inc.) and stained with 2% uranyl acetate or 5 minutes after washing twice with filtered Milli-Q water. The grid was dried and analyzed by Tecnai T20 TEM with 120 kV electron beam.
Cell Viability Assay
Neuro2A cell line (ATCC® CCL-131™) was cultured in DMEM (Invitrogen), supplemented with 10% FBS and 100 μg/mL of penicillin-streptomycin. Ten thousand cells/well were seeded in 96-well plates. Melatonin (Sigma) was added at different concentrations, with 5% DMSO as control at the final volume of 100 μL. Tau aggregates (10 μM) were added separately and along with various concentrations of Melatonin (from 0.1 to 100 μM) and incubated for 24 hours at 37°C. The MTT (Thiazolyl blue tetrazolium bromide) reagent (Sigma) was added at the final concentration of 0.5 mg/mL in each well and incubated for 3 hours at 37°C. The formazan end product was solubilized by adding DMSO and the absorption was measured at 570 nm in spectrophotometer (Infinite® 200 M PRO, Tecan).
Lactate Dehydrogenase (LDH) Assay
Aggregates induce cellular toxicity and alter membrane permeability, which leads to the leakage of cytosolic enzyme LDH (Flach et al., 2012). Neuro2A cells were treated with 10 μM of aggregated Tau and different concentrations (from 1 to 50 μM) of Melatonin together for 24 hours. The cell-free media were collected from each well and LDH leakage was measured by the formation of formazan compound, which is directly proportional to the cytotoxicity as per manufacturer's protocol (Pierce™ LDH Cytotoxicity Assay Kit: 88953).
Detection of Intracellular ROS Production by DCFDA Assay
In order to check intracellular ROS production by Tau aggregates, Neuro2A cells were treated individually and in combination with 1 and 10 μM concentrations of Melatonin and 10 μM of Tau aggregates for 1 hour along with 5% DMSO as a positive control. Then, the cells were washed thrice with PBS and incubated with 25 μM DCFDA (Sigma) for 30 minutes. Intracellular ROS cleaves DCFDA and the amount of DCF fluorescence is directly proportional to ROS production, which was detected by Flow cytometry (BD Accuri C6). The fluorescence positive cell population was selected by live-dead gating (>80% from the whole population) and then acquired 50,000 cells through the FITC channel (Excitation/Emission: 488/530 nm). The relative fluorescence of the test groups was calculated by subtracting the values of autofluorescence of untreated cell control.
Caspase Assay
Caspase-3 activation can be quantified by fluorometric assay in which a signal peptide is linked with a fluorophore molecule (Z-DEVD-AMC substrate). Okadaic acid (OA) is an inducer of global cellular protein phosphorylation and apoptosis, leading to neuronal death (Suuronen et al., 2000, Lavrik et al., 2005). Neuro2A cells were treated with Tau aggregates (10 μM) and OA (25nM) with Melatonin (50 μM), and incubated for 6 hours. Then, the cells were harvested, lysed and mixed with fluorophore-conjugated signal peptide substrate (EnzChek™ Caspase-3 Assay Kit: E13184) and incubated for 30 minutes. Caspase 3 cleaves the signal peptides and the amount of released fluorophores is measured by Excitation/Emission at 485/535 nm, which is proportional to the apoptotic activity.
Western Blot
To study the protein expression level of GSK3β and pGSK3β (phosphorylated glycogen synthase kinase 3β; Ser9), we treated Neuro2A cells with okadaic acid (25 nM) and Melatonin (50 μM) separately and together for 24 hours. The cells were washed with PBS and lysed with RIPA buffer (Invitrogen) and cell lysate were subjected to western blot with anti GSK3β monoclonal antibody (MA5-15109) (1:2500), anti-p-GSK3β monoclonal antibody (MA515109) (1:2000) and anti Nrf2 polyclonal antibody (PA5-68817) (1:1000) with β-tubulin (MA516308) (1:5000), α-Actin (Sigma: A2066) (1:2500) as internal control. Then, the bands' intensity was quantified by using BIORAD Quality one 4.6.6 software. The band-density of the treated group was compared with its corresponding untreated control group and normalized with specific house-keeping gene control (β-tubulin or α-Actin) (n = 2). The pGSK3β levels were compared with total GSK3β level among various treatment groups while the total GSK3β levels were compared to α-Actin level. Similarly, The Nrf2 level was compared with β-tubulin loading control in four different treatment conditions. Then, the relative fold changes were plotted with respective proteins and treated groups.
Immunofluorescence Assay
The level of Tau phosphorylation (pT181-Tau and AT100-Tau) upon okadaic acid (25 nM) treatment and the role of Melatonin (50 μM) on cellular kinase- GSK3β were checked by immunofluorescence study along with oxidative stress response transcription factor Nrf2. Neuro2A cells were treated with OA and Melatonin together and separately for 24 hours. Then, the cells were washed with PBS thrice and fixed with chilled absolute methanol for 15 minutes and permeabilized with 0.2% TritonX-100. The cells were stained with Phospho-Tau Ser212/Thr214 (MN1060) (AT100 1:100), Phospho-Tau Thr181(5H9L11) (1:100), p-GSK3β (Ser9) (1:100), GSK3β (1:100), Nrf2 (1:100) and T46 (13-6400) (1:250) antibody for overnight in 2% horse serum. Then, Alexa fluor-secondary antibody- anti-mouse secondary antibody conjugated with Alexa Fluor-488 (A-11001), Goat anti-Rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody with Alexa Fluor 555 (A-21428) were allowed to bind p-Tau, p-GSK3β, T46, total GSK3β and Nrf2 along with total Tau for 1 hour along with nuclear stain-DAPI (300 nM). The microscopic images were taken in Zeiss Axio observer 7 with Apotome 2.0 fluorescence microscope at 63X oil immersion. The quantification was done using Zen 2.3 software and the mean fluorescence intensity was determined and plotted for different test groups.
Expression Profile Study by Quantitative Real-Time PCR
The microglial cell line N9 (CVCL_0452) was cultured in RPMI 1640 media (Invitrogen), supplemented with 10% FBS and 100 µg/ml penicillin-streptomycin (Das et al., 2020). To study the immune potential of Melatonin on microglial activation and inflammation, N9 cells were treated with Tau aggregates (10 μM), Melatonin (50 μM) and okadaic acid (25 nM) as a positive control for 6 hours. Then the cells were washed PBS and total RNA was isolated by the conventional TRIZOL reagent (Invitrogen), chloroform and isopropanol extraction procedure (Rio et al., 2010). The RNA pellets were washed with 80% ethanol, dissolved in DEPC treated water, and proceeded for cDNA synthesis (First-strand cDNA synthesis kit: K1612) using the oligo-dT primer. The expression level of various cytokines (TNF-α, IL1β, IL10, IL4, TGF-β [Tumor growth factor β]), inflammatory marker (COX2, NOS1, Arg1) involved in Tauopathy were checked by real-time PCR (Maxima SYBR Green/Fluorescein qPCR Master Mix (2X). Similarly, the expression of protein kinases (GSK3β, MAP3K [MAP kinase kinase kinase]) and NFkB component (P65) were checked by qRT-PCR (Table 1). The fold change was calculated by the ΔΔCT method with respect to house-keeping GAPDH control.
Table 1.
Primer List Used for qRT-PCR Expression Profile Study.
| Target gene | Primer sequence (5′-3′) |
|---|---|
| TNF α | Fw- AACCTCCTCTCTGCCGTCAA Rv- CTCCAAAGTAGACCTGCCCG |
| IL 1β | Fw- TCTTTGAAGTTGACGGACCCC Rv- GCTTCTCCACAGCCACAATG |
| IL 10 | Fw-TAACTGCACCCACTTCCCAG Rv- GGGGCATCACTTCTACCAGG |
| IL 4 | Fw- CGGCATTTTGAACGAGGTCA Rv- TGCAGCTCCATGAGAACACT |
| TGF β | Fw- AGGAGACGGAATACAGGGCT Rv- CATGAGGAGCAGGAAGGGC |
| COX 2 | Fw- TTGGAGGCGAAGTGGGTTTT Rv- TCTAGTCTGGAGTGGGAGGC |
| NOS 1 | Fw- GCCACACTTCTCCTCACACA Rv- AGCCTGTATTCGGTTGAGCC |
| Arg 1 | Fw- CGTGTACATTGGCTTGCGAG Rv- TCGGCCTTTTCTTCCTTCCC |
| GSK 3β | Fw- GTTCTCGGTACTACAGGGCAC Rv- CCACCAACTGATCCACACCA |
| MAP3K | Fw- GCTTATCAACACCACCTGCG Rv- AGAATGCAGCCCACAGACC |
| P65 | Fw- GGCTACACAGGACCAGGAAC Rv- GGTCTGGATTCGCTGGCTAA |
Statistical Analysis
Each experiment was performed two times and all measurements were taken in triplicate. Statistical analysis for cytotoxicity assay, DCFDA fluorescence assay, caspase assay, western blot and microscopic quantification were performed by using one-way ANOVA in Microsoft excel software and the statistical significance was considered at the 5% level of significance. For cytotoxicity, LDH, DCFDA and caspase assay, the Tau aggregates, okadaic acid and Melatonin treated groups were compared with untreated cell control. While the ‘okadaic acid and Melatonin’ and ‘Tau aggregates and Melatonin’ group were compared with only okadaic acid-treated and Tau aggregates-treated groups, respectively. For pGSK3β-GSK3β and Nrf2 immunofluorescence study, 4 cells were analyzed from each field and then 7 fields from triplicate were analyzed for microscopic analysis (n = 28). For the pT181-Tau immunofluorescence study, 4 cells were analyzed from each field and then 11 fields from triplicate were analyzed for microscopic analysis (n = 44). The p-values were calculated and represented as p<0.05 as *, p<0.001 as ** and p<0.0001 as ***.
Results
Melatonin Reduces Tau-Mediated Cytotoxicity
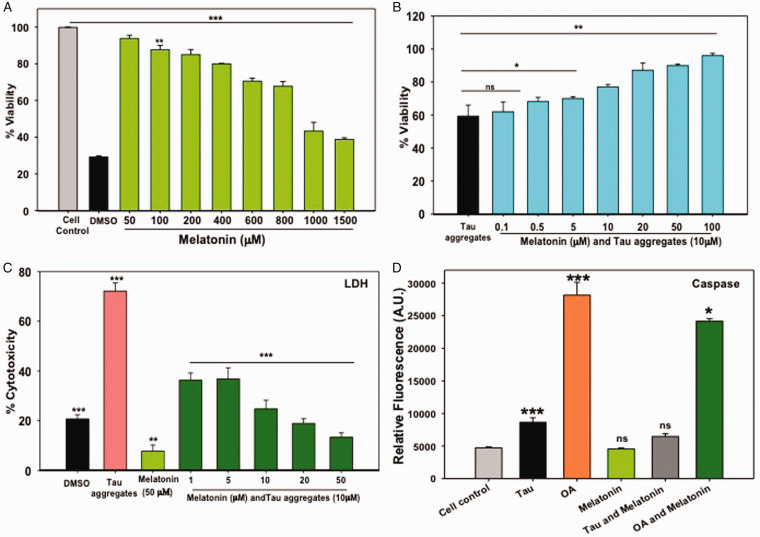
Extracellular deposition of amyloid-β plaques and intracellular NFTs of Tau induce neuronal toxicity, which leads to cognitive impairment due to synaptic loss in AD. Melatonin is known as a neuroprotectant, improving mitochondrial functions, antioxidant, immune modulator (Galano et al., 2014, Reiter et al., 2016) and restore cognitive function (Cardinali et al., 2012). In our study, it was observed that Melatonin reduced the Tau-mediated cytotoxicity in a concentration-dependent manner. Melatonin showed 50% viability at 1 mM concentration on Neuro2A cells by MTT assay (Figure 1A) and the phase-contrast study depicted the neuronal cell extensions even at higher concentrations of Melatonin (Suppl. Fig. 1A). Upon treatment of 10 μM of Tau aggregates, Neuro2A showed only 60% viability, but, Melatonin at 20 μM concentration rescued the viability up to 80% in Tau-exposed neurons (Figure 1B). The cytotoxicity was completely reversed at 200 μM concentration of Melatonin (Suppl. Fig. 1A).
Figure 1.
Cytotoxicity and Caspase Activity Assay. A: Melatonin showed cytotoxicity at only higher concentrations (>1 mM) on Neuro2A cells, as found by MTT formazan production. B: Pre-formed Tau aggregates at 10 μM showed 60% viability on Neuro2A cells, but Melatonin increased the viability of Neuro2A cells in a concentration-dependent manner. C: Cytotoxicity study was confirmed by LDH assay. Tau aggregates (10 μM) induced 70% cytotoxicity, whereas Melatonin treatment reduced the Tau aggregates-mediated cytotoxicity in a concentration-dependent way. D: OA induced high caspase activity as compared to Tau aggregates treatment. Melatonin treatment reduced the OA induced caspase activity effectively, but Tau mediated caspase activity remains unaltered. Values were given as mean + SEM. * corresponds to p-values of test groups compared with untreated and positive control (*p < 0.05; **p < 0.01, ***p < 0.001).
The leakage of cytoplasmic enzyme LDH is a determinant of cytotoxicity and membrane damage. Tau aggregates treatment on Neuro2A cells resulted in 68% cytotoxicity, while the 10 μM concentration of Melatonin reduced 50% Tau-mediated cytotoxicity (Figure 1C). The pelleting assay and SDS-PAGE analysis have shown the formation of mature Tau fibrils with a heterogeneous mixture of aggregates of >150–250 kDa and fibrillar aggregates as seen by TEM analysis (Suppl. Fig. 1B, C).
Anti-Apoptotic Role of Melatonin
Caspases-3 is the central molecule for the aspartate-guided cleavage of the target protein in both intrinsic (mitochondrial) and extrinsic (Death ligand) pathway of apoptosis. Previous reports suggested that caspase-3 can cleave APP (amyloid precursor protein), which leads to further aggregation (Gervais et al., 1999) and Melatonin prevents the intrinsic pathway of apoptosis in neurodegenerative diseases (Wang, 2009). Here, Tau aggregates and OA (positive control) induced two-fold and five-fold caspase 3 activity, respectively, on Neuro2A cells as compared to untreated control. Melatonin can reduce the OA-mediated caspase activation at 50 μM concentration while Tau aggregates mediated apoptosis remain unaltered by Melatonin (Figure 1D).
Melatonin Reduced GSK3β Expression but Not p-GSK3β (Ser9) Level
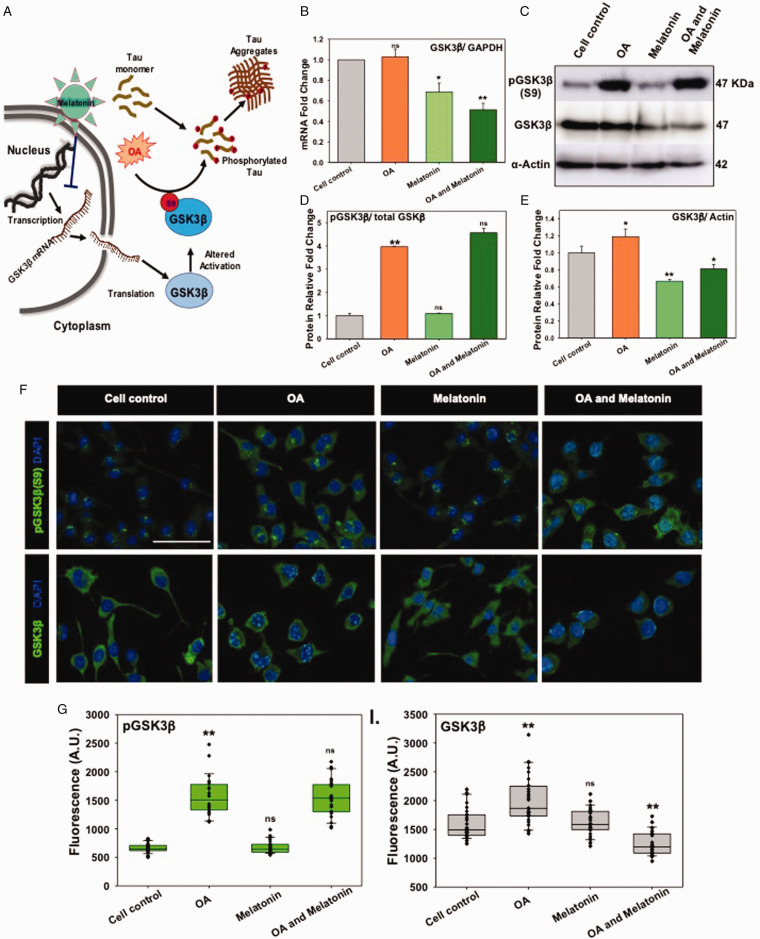
Modified Tau dissociates from microtubules and becomes aggregated in the cytosol during AD (Avila, 2006). GSK3β, a serine-threonine kinase, with altered activation state, mediates hyperphosphorylation of Tau in AD (Sun et al., 2015). To test the role of Melatonin on GSK3β-mediated Tau-phosphorylation (Figure 2A), Neuro2A cells were treated with OA, which inhibits the protein phosphatase 2A and thereby induces the phosphorylation of intracellular Tau. The RT-PCR study has revealed that Melatonin downregulated the GSK3β mRNA level significantly alone as well as in OA-stressed neuronal cells (Figure 2B). As the OA exposure can induce the global phosphorylation in cells, the p-GSK3β (Ser9) level was found to be high as compared to total GSK3β level by western blot study (Figure 2C and D). Melatonin treatment on Neuro2A cells has shown a reduced protein level of GSK3β, but the phosphorylation at Ser9 on GSK3β as an altered activated state remained invariable (Figure 2D and E). Immunofluorescence study depicted that the upregulation of total GSK3β and phospho-GSK3β level upon OA exposure (Figure 2F), but only total GSK3β level was reduced in Melatonin-treated and OA-stressed neurons (Figure 2G and H). The Melatonin exerts its effect by reducing the transcriptomics level of GSK3β, without altering the GSK3β (Ser9) activation state. Hence, the Melatonin-reduced GSK3β expression can be a potential target in the reduction of Tau hyperphosphorylation in AD.
Figure 2.
Melatonin Altered GSK3β Expression but Not Activation. A: In AD, Tau becomes hyperphosphorylated and subsequently deposits as aggregates in neurons. OA has been proven as a model to induce Tau phosphorylation via PP2A inhibition and enhancement of overall kinase activity. Melatonin has been proposed to block the GSK3β-mRNA expression and total protein level. B: Melatonin reduced the total-GSK3β mRNA expression level on Neuro2A cells upon OA treatment by qRT-PCR. C and D: OA has induced the Ser9 phosphorylation of GSK3β due to the overall enhancement of global phosphorylation, whereas the level of total GSK3β decreased slightly. E: Western blot densitometric quantification showed a decreased level of total GSK3β in Melatonin-treated Neuro2A cells. F: Immunofluorescence study depicted the altered level of only total GSK3β protein, but GSK3β (phopsho Ser9) remained invariant. G and H: The relative fluorescence level was plotted for Melatonin and OA treated group alone and together. * corresponds to p-values of test groups compared with untreated control (*p < 0.05; **p <0.01, ***p<0.001). scale bar: 50 μm.
Melatonin Decreases pTau Level
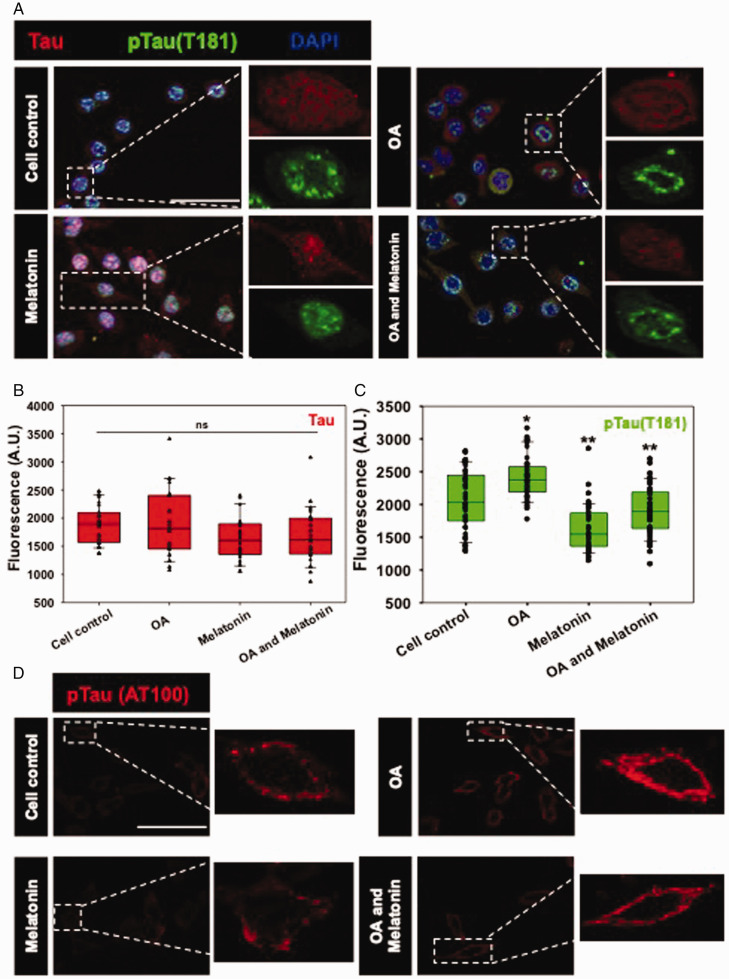
Tau undergoes hyperphosphorylation by the overactivation of cellular kinases in AD condition, where Tau loses its affinity towards microtubule and remains freely diffusible in the cytosol. The pTau forms oligomeric seed species by lowering the intermolecular interaction and becomes aggregated as insoluble inclusions. Altered activated-GSK3β (Tyr216) induces the phosphorylation of Tau at different Serine/Threonine residues (Yuan et al., 2004, Noble et al., 2005, Koh et al., 2008) and changes its subcellular localization from axons to cell bodies and even at nucleus in AD (Lu et al., 2013). The pTau (Thr181) level was found to be upregulated upon OA exposure, while the distribution of pTau became concentrated at the nuclear periphery (Figure 3A and B). Melatonin alone significantly depleted the nuclear pTau (Thr181) level in Neuro2A cells as compared to the untreated control, while the OA-induced pTau level also became downregulated by Melatonin (Figure 3A). The phosphorylation at AT100 epitope of Tau is a signature of amyloidogenic Tau phosphorylation, which was observed to localize around the cytoplasmic membrane as neuronal puncta (Figure 3D). The exposure of OA-induced the punctate-AT100 Tau level in the Neuro2A cell membrane, which was reduced significantly by Melatonin (Suppl. Fig. 3).
Figure 3.
Melatonin reduces OA-mediated Tau phosphorylation. A: Tau phosphorylation at pThr181 is one of the epitopes found in the AD condition, which is acted upon by GSK3β. Phospho-Tau (Thr181) was found to be localized into the nucleus, while OA exposure resulted in concentrating the particular phospho-Tau epitope only in the nuclear periphery. B and C: Quantification of mean fluorescence intensity showed a decrease in Phospho-Tau (pThr181) level by Melatonin treatment. D: Phospho-Tau (AT100) also showed its localization in the membrane periphery, induced by OA. Melatonin rescued the AT100-phosphoTau level in OA treated Neuro2A cells. (Scale bar: 50 μm.)
Melatonin Quenches Intracellular ROS Production and Mediates Nrf2 Translocation Into the Nucleus
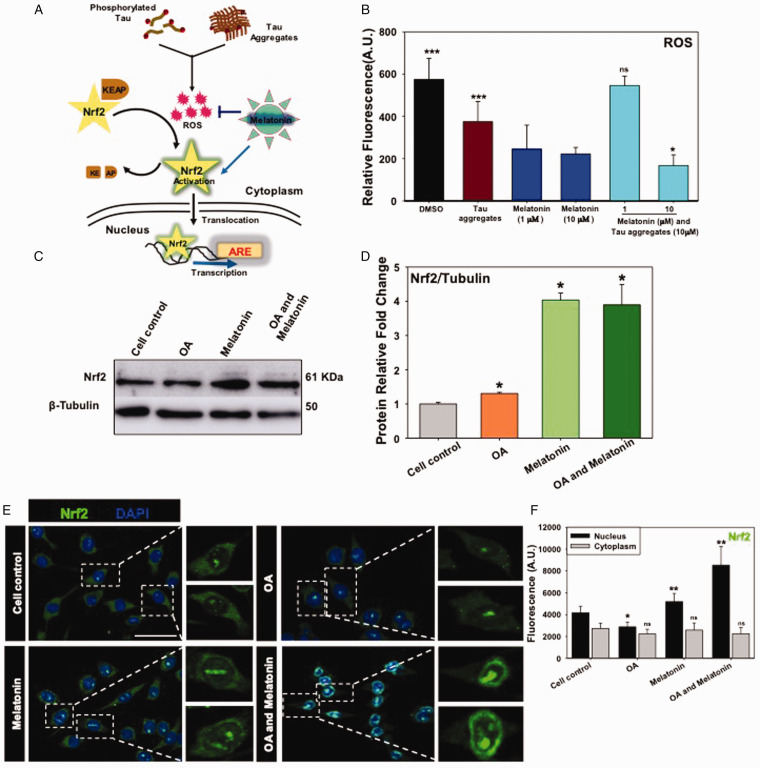
In order to check the antioxidant property of Melatonin, the intracellular ROS quenching was analyzed by DCFDA fluorescence assay on Neuro2A cells upon Tau aggregates-exposure. Tau treatment increased the intracellular ROS level to 700 A.U., which was similar to the positive control DMSO (∼500 A.U). Melatonin at 10 μM concentration reduced almost 50% of the ROS level in Tau-exposed Neuro2A cells (Figure 4B). Together, Melatonin shows the intracellular ROS scavenging property and potency in rescuing the neurons from Tau aggregates-mediated oxidative damage.
Figure 4.
Melatonin Quenches ROS Production and Nrf2 Translocation Into the Nucleus. A: Intracellular phosphorylated Tau and Tau aggregates have the potential to induce ROS production. Nrf2-KEAP1 complex present in cytosol as inactive form, but oxidative stress induces the dissociation of KEAP from Nrf2. Free Nrf2 becomes activated and translocates into the nucleus for transcription of the antioxidant responsive element (ARE) as global production of anti-oxidant enzymes and subsequent impelled proteostasis. B: Tau aggregates treatment-induced intracellular ROS level while Melatonin at 10 μM concentration reduced the intracellular ROS level (DCFDA fluorescence) significantly as detected by FACS. C and D: Melatonin rescued the neurons from oxidative damage by increasing the protein level of Nrf2 extensively as compared with β-tubulin as a loading control in OA-stressed Neuro2A cells. E and F: Melatonin treatment was associated with induced Nrf2 level in the nucleus while the complete nuclear translocation was evident along with OA-stress conditions as observed by immunostaining. (Scale bar: 50 μm.)
Under physiological condition, Nrf2 is a short-lived protein, but upon activation, Nrf2 becomes stabilized and translocates into the nucleus to subsequently activate the transcription of antioxidant enzymes and anti-inflammatory genes (Figure 4A). In western blot analysis, Melatonin induced the Nrf2 expression effectively even in the presence of OA stress, which suggests that Melatonin can mediate the Nrf2-driven anti-oxidant function in neuronal cells (Figure 4C and D). Additionally, the Nrf2 level was found to be increased, especially in the nucleus by Melatonin treatment, while the nuclear translocation of Nrf2 was maximum in OA and Melatonin treatment group as observed by immunofluorescence study. Together, these results emphasize the Nrf2-mediated oxidative stress response and corresponding rescuing effect by Melatonin in Neuro2A cells (Figure 4E and F).
Anti-Inflammatory Response by Melatonin in N9 Microglia
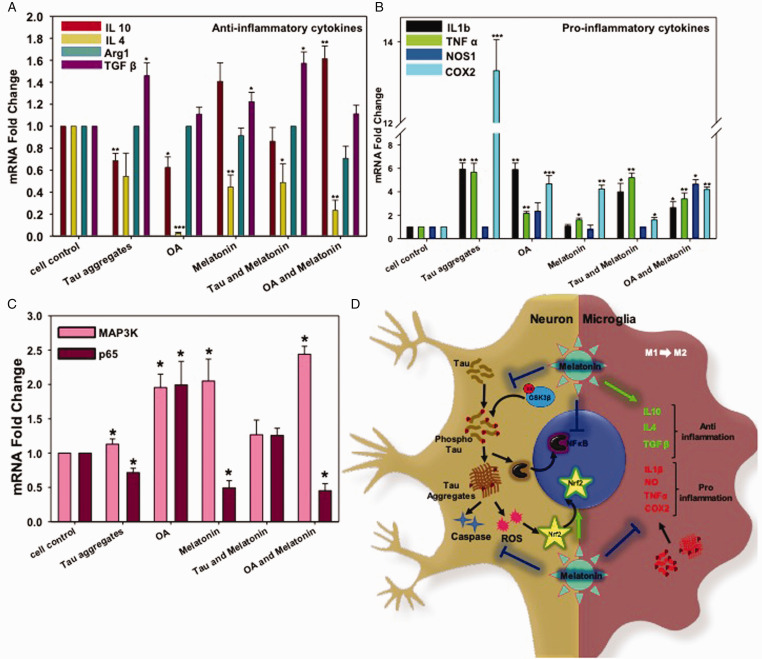
Melatonin is able to interact with the immune system, especially regulating the anti-inflammatory state. Melatonin prevents the lipid peroxidation in the mitochondrial membrane (Reiter et al., 2001, Altun and Ugur-Altun, 2007), and has shown to reduce the activation of NFκB (Camello-Almaraz et al., 2008). For anti-inflammatory function, we checked the cytokine profile of Melatonin on the microglial cell line (N9). The OA and Tau-exposed N9 cells have shown to downregulate the expression of IL-4 and IL-10 significantly, while the Melatonin induced the IL-10 mRNA expression in OA-stressed N9 cells (Figure 5A) which may correlate with anti-inflammation (Emmerich et al., 2012) and opsonization of Tau aggregates. Similarly, Melatonin also increased TGF-β expression upon Tau-exposure (Figure 5A), which might reduce the oxidative stress and acts as a chemo-attractant for the infiltrating macrophages with increased phagocytic activity (Letterio and Roberts, 1998). Additionally, TGF-β secretion by microglia leads to initiate the neuronal repair upon Tau exposure through the transcription of MAP3K cellular kinase. As Melatonin induces TGF-β expression, it may probably induce MAP3K-mediated (Figure 5C) axonal survival in neurons (Walker et al., 2017). Tau and OA treatment significantly reduced IL-4 expression (Figure 5A), but Melatonin restored the levels slightly, which determines a slow induction of the M2 phenotype of neuroprotective microglia.
Figure 5.
Real-Time Expression of Pro- and Anti-Inflammatory Cytokines in N9 Cells. A: Melatonin-induced the level of the anti-inflammatory cytokine (TGFβ and IL10) alone and together with either Tau aggregates or OA. B: OA and Tau aggregates induced pro-inflammatory cytokines (IL 1β, TNFα, COX2) excessively, but Melatonin rescued the inflammatory damage by reducing the expression of COX2. IL1β expression level was also brought down by Melatonin treatment upon Tau and OA exposure; however, TNFα mRNA level remains unchanged. C: the mRNA expression level of p65 (NFκB) increased upon Tau and OA treatment, whereas; Melatonin reduced the P65 level compared to untreated group. The MAP3K expression level was increased by 2.5 times upon Melatonin treatment, which is related to neuronal survival. Values were given as mean + SEM. * corresponds to test groups compared with untreated control (*p < 0.05; **p <0.01, ***p<0.001). D: Activation of cellular kinase leads to Tau phosphorylation and aggregates accumulation, which in turn activates ROS production and Caspase-apoptotic signal in neurons. Aggregated Tau can be released through axons, which are eventually uptaken by microglia and returned into the ‘Activation' state. Activated microglia enlarges its extension and produce excess pro-inflammatory cytokines. But Melatonin treatment reduces the activated state of microglia and allows to secrete more anti-inflammatory cytokines, which can manage the burden of aggregates by increased phagocytic activity. Melatonin reduces NFκB activation in inflammation, but MAP3K activation and transition towards the M2 phenotype of microglia lead to axonal repair and survival.
Tau aggregates and OA induced the amount of pro-inflammatory cytokines like- IL-1β, TNF-α, and COX2 expression (Figure 5B). TNF-α is an acute-phase protein, which simultaneously activates NFκB and MAPK, p38, ERK signaling cascades and phosphorylates several cellular proteins, transcription factors related to inflammation. (Wajant et al., 2003) This can be correlated with the level of Tau phosphorylation and overactivation of microglia in AD. Melatonin was able to reduce the level of IL-1β in response to Tau phosphorylation and deposition by N9 cells, while the TNF-α level can only be decreased upon OA exposure. OA induces p65 mRNA expression, which is reduced by treatment of Melatonin in Neuro2A cells. This might be due to the downregulation of NFκB-mediated neuroinflammation in response to Tau hyperphosphorylation (Figure 5C). COX is the enzyme involved in prostaglandin biosynthesis from arachidonic acid and is the main target for the non-steroidal anti-inflammatory drugs (NSAIDs). Tau aggregates and OA treatment induced COX2 expression, which correlates with the acute inflammatory state in microglia. Melatonin reduced the COX2 level significantly, which suggests its effect on microglial patho-protein response and anti-inflammation but not when Tau phosphorylation occurs (Figure 5B). IL-1β is a pyrogenic cytokine that activates the COX in CNS, leading to inflammatory pain. Melatonin reduced IL-1β level in Tau and OA treated groups similar to COX2. Collectively, it is prominent that Melatonin not only activates the anti-inflammatory state in microglia but also reduces the pro-inflammatory molecules in AD (Figure 5D).
Discussion
AD is a multifactorial disease that includes the aggregation of β-amyloid protein as plaques in the brain. An altered signaling cascade leads to abnormal modifications of intracellular protein Tau and its aggregation in the form of NFTs (Mandelkow and Mandelkow, 2012). Excessive aggregates accumulation affects the neuro-trafficking and neurotransmitter release, leading to axonal blockage and attenuated neuronal plasticity (Guo and Lee, 2014). Extracellular protein aggregates activate microglia with increased production of ROS/NO (Bedard and Krause, 2007), inflammatory cytokines, chemokines and complements (Schetters et al., 2018) which, ultimately results in the loss of neuronal synapses by phagocytosis (Mirbaha et al., 2018). Age-associated neurological disorders are often associated with impaired circadian rhythm due to the shortage of pineal-produced Melatonin levels (Zhou et al., 2003). Melatonin has been identified as a mighty lead as natural anti-oxidant, anti-cancer, an anti-inflammatory molecule, enzyme regulator, metabolic-energy sustainer, epigenetic coordinator and aggregation inhibitor, etc. (Balmik and Chinnathambi, 2018). Previously, our group has reported that Melatonin can prevent the Tau aggregation via interacting with the repeat domain and the Melatonin-induced Tau oligomeric species are non-toxic to Neuro2A cells (Balmik et al., 2019). Here, we found that Melatonin can reduce the Tau aggregates-exerted neuro-toxicity at lower concentration and remain non-toxic even at higher concentrations. Matured Tau fibril can lead to plasma membrane leakage and mediates the subsequent spreading of Tau seeds from affected neurons to others in Tauopathy (Guo and Lee, 2014). In our study, we observed the membrane leakage in neurons upon Tau fibrils exposure by LDH leakage. But the membrane leakage was restrained by Melatonin, which signifies its role in retaining the membrane integrity of neurons in AD. We perceived that Melatonin could prevent the OA-induced apoptosis in neurons, but when Tau aggregates were exposed to neuronal cells, Melatonin can not reduce the caspase3 activation. This emphasizes that Melatonin can prevent the intrinsic apoptotic signal induced by Tau phosphorylation, but the trigger from the extrinsic pathway via aggregates accumulation can not be altered in damaged neurons. Hyperphosphorylated Tau is one of the early pathological scenario of AD, which is mediated by the over-activation of cellular kinases (Sun et al., 2015). Melatonin regulates kinase activity by interfering with intermediate signaling cascade Akt/PI3K (Ali and Kim, 2005) or receptor-mediated mechanism (Wang et al., 2004). Phosphorylated Tau was found to co-localize with Aβ (Amyloid-β) on synaptosomal membrane compartments (Fein et al., 2008). Our study showed that Melatonin had reduced the level of nuclear phospho-Tau (pT181), which were found to be upregulated and concentrated around the nuclear periphery upon OA-exposure. Similarly, the level of AT100 phosphoTau was found to be increased due to OA-mediated hyperphosphorylation and observed to be localized as neuronal puncta. Melatonin has shown to reduce the membrane-associated AT100-pTau level, even upon the induction with OA. The GSK3β is an important kinase which is involved in Tau hyperphosphorylation upon altered activation in AD (Koh et al., 2008). Here, Melatonin treatment mediated the downregulation of GSK3β mRNA and, eventually, protein level. But, the functional activity of GSK3β marked by pSer9, which represents its downregulated state, remained unaffected by Melatonin (Yuan et al., 2004). Altogether, Melatonin mitigates the nuclear as well as membrane-associated Tau phosphorylation and also alleviates the GSK3β expression in the scenario of Tau accumulated neurodegeneration.
Being a global transcriptional activator, Nrf2 actively transcribes antioxidant response elements (ARE) and functions to expand the level of different antioxidant enzymes like- catalase, superoxide dismutase (SOD), peroxidase, the detoxifying enzyme in xenobiotic metabolism to oxidative stress (Murphy and Park, 2017). Nrf2 also plays a crucial role in microglial phenotypic determination. Depletion of Nrf2 induces inflammatory burst, NFκB activation, and reduced phagocytic activity in primary microglia upon α-synuclein exposure. Nrf2−/− mice have shown an impaired ubiquitin-proteasomal system in α-synuclein overload as compared to WT (Lastres-Becker et al., 2012). Hence, the activation of global antioxidant transcription factor-Nrf2, along with the neurotrophic factor, can be a potential therapeutic strategy to combat AD (Murphy and Park, 2017). We found that Melatonin quenched ROS production and mediated anti-oxidation via activation and subsequent nuclear translocation of Nrf2 in neurons. Neuroinflammation is one of the important events in AD mainly mediated by glia through various small messenger molecules, complement factors, and effector enzymes. Melatonin acts phenomenally as an immuno-modulator where it prompted IL-10 and TGF-β activation related to anti-inflammation and MAP3K-mediated axonal repair. Melatonin also downregulated the markers of inflammatory burst involved in neurodegenerative diseases, namely COX2, TNF-α, and IL-1β. Altogether, Melatonin performs the multi-faceted role in the mediation of neuroprotection, reducing Tau phosphorylation and the regulation of neuroinflammation.
Conclusions
Melatonin plays a pleiotropic role in Tauopathy, by reducing Tau phosphorylation, dissolution aggregates-mediated toxicity, reducing membrane leakage in neurons. It also mediates the anti-oxidation and anti-apoptotic function by quenching free radicals and deactivating Caspase-3 respectively. Melatonin also rescued OA-induced Tau phosphorylation by downregulating GSK3β expression and Nrf2 activation-translocation. Melatonin altered microglial phenotype from pro-to anti-inflammatory state in Tau-mediated neurodegeneration. Thus, owing to its multiple protective functions in neuronal health, Melatonin can be employed in designing therapies against Taupathies in combination with other potent drugs.
Supplemental Material
Supplemental material, sj-pdf-1-asn-10.1177_1759091420981204 for Melatonin Reduces GSK3β-Mediated Tau Phosphorylation, Enhances Nrf2 Nuclear Translocation and Anti-Inflammation by Rashmi Das, Abhishek Ankur Balmik and Subashchandrabose Chinnathambi in ASN Neuro
Acknowledgments
Tau constructs were kindly gifted by Prof. Roland Brandt from University of Osnabruck, Germany. The authors acknowledge Dr. H V Thulasiram for q-real time PCR facility. Special thanks to Mr. Ashish Kumar for helping in expression profile study. Special thanks to Mr. Tushar Dubey for proof-reading the article. The authors gratefully acknowledge Professor H. V. Thulasiram, Chemical Biology group at the Division of Organic Chemistry, CSIR-National Chemical Laboratory for his excellent Molecular Biology Lab facilities and for his constant support.
Footnotes
Author Contributions: R. D., A. B. and S. C. conducted most of the experiments, analyzed the results, and wrote the paper. S. C. conceived the idea of the project, designed, provided resources, supervised and wrote the paper. We are grateful to Chinnathambi's lab members for their critical readings and fruitful discussions. All authors approved the final manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Rashmi Das acknowledges the fellowship from University Grant Commision (UGC) India. Abhishek Ankur Balmik acknowledges Shyama Prasad Mukherjee fellowship (SPMF) from Council of Scientific Industrial Research (CSIR), India. This project is supported by in-house CSIR-National Chemical Laboratory grant MLP029526.
ORCID iD: Subashchandrabose Chinnathambi https://orcid.org/0000-0002-5468-2129
Supplemental material: Supplemental material for this article is available online.
References
- Ali T., Kim M. O. (2005). Melatonin ameliorates amyloid beta-induced memory deficits, tau hyperphosphorylation and neurodegeneration via PI 3/Akt/GSk3β pathway in the mouse hippocampus. J Pineal Res, 59(1), 47–59. [DOI] [PubMed] [Google Scholar]
- Altun A., Ugur-Altun B. (2007). Melatonin: Therapeutic and clinical utilization. Int J Clin Pract, 61(5), 835–845. [DOI] [PubMed] [Google Scholar]
- Avila J. (2006). Tau phosphorylation and aggregation in Alzheimer's disease pathology. FEBS Lett, 580(12), 2922–2927. [DOI] [PubMed] [Google Scholar]
- Balmik A. A., Chinnathambi S. (2018). Multi-faceted role of melatonin in neuroprotection and amelioration of Tau aggregates in Alzheimer's disease. J Alzheimer's Dis, 62(4), 1481–1493. [DOI] [PubMed] [Google Scholar]
- Balmik A. A., Das R., Dangi A., Gorantla N. V., Marelli U. K., Chinnathambi S. (2019). Melatonin interacts with repeat domain of Tau to mediate disaggregation of paired helical filaments. Biochim Biophys Acta Gen Subjects, 1864, 129467. [DOI] [PubMed] [Google Scholar]
- Barghorn S., Biernat J., Mandelkow E. (2005). Purification of recombinant tau protein and preparation of Alzheimer-paired helical filaments in vitro. Methods Mol Biol, 299, 35–51. [DOI] [PubMed] [Google Scholar]
- Bedard K., Krause K.-H. (2007). The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev, 87(1), 245–313. [DOI] [PubMed] [Google Scholar]
- Brunello C. A., Merezhko M., Uronen R.-L., Huttunen H. J. (2020). Mechanisms of secretion and spreading of pathological tau protein. Cell Mol Life Sci, 77(9), 1721–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camello-Almaraz C., Gomez-Pinilla P. J., Pozo M. J., Camello P. J. (2008). Age-related alterations in Ca2+ signals and mitochondrial membrane potential in exocrine cells are prevented by melatonin. J Pineal Res, 45(2), 191–198. [DOI] [PubMed] [Google Scholar]
- Cardinali D. P., Vigo D. E., Olivar N., Vidal M. F., Furio A. M., Brusco L. I. (2012). Therapeutic application of melatonin in mild cognitive impairment. Am J Neurodegen Dis, 1(3), 280. [PMC free article] [PubMed] [Google Scholar]
- Claustrat B., Brun J., Chazot G. (2005). The basic physiology and pathophysiology of melatonin. Sleep Med Rev, 9(1), 11–24. [DOI] [PubMed] [Google Scholar]
- Das R., Balmik A. A., Chinnathambi S. (2020). Phagocytosis of full-length Tau oligomers by actin-remodeling of activated microglia. J Neuroinflam, 17(1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David D. C., Hauptmann S., Scherping I., Schuessel K., Keil U., Rizzu P., Ravid R., Dröse S., Brandt U., Müller W. E., Eckert A., Götz J. (2005). Proteomic and functional analysis reveal a mitochondrial dysfunction in P301L tau transgenic mice. J Biol Chem, 280(2), 23802–23814. [DOI] [PubMed] [Google Scholar]
- Emmerich J., Mumm J. B., Chan I. H., LaFace D., Truong H., McClanahan T., Gorman D. M., Oft M. (2012). IL-10 directly activates and expands tumor resident CD8+ T cells without de novo infiltration from secondary lymphoid organs. Cancer Res, 72(14), 3570. [DOI] [PubMed] [Google Scholar]
- Fein J. A., Sokolow S., Miller C. A., Vinters H. V., Yang F., Cole G. M., Gylys K. H. (2008). Co-localization of amyloid beta and tau pathology in Alzheimer's disease synaptosomes. Am J Pathol, 172(6), 1683–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flach K., Hilbrich I., Schiffmann A., Gaertner U., Krueger M., Leonhardt M., Waschipky H., Wick L., Arendt T., Holzer M. (2012). Tau oligomers impair artificial membrane integrity and cellular viability. J Biol Chem, M112, 396176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galano A., Tan D. X., Reiter R. J. (2014). Cyclic 3-hydroxymelatonin, a key metabolite enhancing the peroxyl radical scavenging activity of melatonin. RSC Adv, 4(10), 5220–5227. [Google Scholar]
- Gehrmann J., Matsumoto Y., Kreutzberg G. W. (1995). Microglia: Intrinsic immuneffector cell of the brain. Brain Res Rev, 20(3), 269–287. [DOI] [PubMed] [Google Scholar]
- Gervais F. G., Xu D., Robertson G. S., Vaillancourt J. P., Zhu Y., Huang J., LeBlanc A., Smith D., Rigby M., Shearman M. S. (1999). Involvement of caspases in proteolytic cleavage of Alzheimer’s amyloid-β precursor protein and amyloidogenic Aβ peptide formation. Cell, 97(3), 395–406. [DOI] [PubMed] [Google Scholar]
- Gilad E., Wong H. R., Zingarelli B., Virág L., O'Connor M., Salzman A. L., Szabó C. (1998). Melatonin inhibits expression of the inducible isoform of nitric oxide synthase in murine macrophages: Role of inhibition of NFκB activation. FASEB J, 12(9), 685–693. [DOI] [PubMed] [Google Scholar]
- Gorantla N. V., Chinnathambi S. (2018). Tau protein squired by molecular chaperones during Alzheimer’s disease. J Mol Neurosci, 66(3), 356–368. [DOI] [PubMed] [Google Scholar]
- Gorantla N. V., Khandelwal P., Poddar P., Chinnathambi S. (2017). Global conformation of tau protein mapped by Raman spectroscopy. Tau Protein (pp. 21–31). Springer. [DOI] [PubMed] [Google Scholar]
- Guo J. L., Lee V. M. (2014). Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat Med, 20(2), 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S., Beja-Glasser V. F., Nfonoyim B. M., Frouin A., Li S., Ramakrishnan S., Merry K. M., Shi Q., Rosenthal A., Barres B. A. (2016). Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science, 352(6286), 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. V. W., Hartigan J. A. (1999). Tau protein in normal and Alzheimer's disease brain: An update. J Alzheimer's Dis, 1(4-5), 329–351. [DOI] [PubMed] [Google Scholar]
- Koh S.-H., Noh M. Y., Kim S. H. (2008). Amyloid-beta-induced neurotoxicity is reduced by inhibition of glycogen synthase kinase-3. Brain Res, 1188, 254–262. [DOI] [PubMed] [Google Scholar]
- Lastres-Becker I., Ulusoy A., Innamorato N. G., Sahin G., Rábano A., Kirik D., Cuadrado A. (2012). α-Synuclein expression and Nrf2 deficiency cooperate to aggravate protein aggregation, neuronal death and inflammation in early-stage Parkinson's disease. Human Mol Genet, 21(14), 3173–3192. [DOI] [PubMed] [Google Scholar]
- Lavrik I. N., Golks A., Krammer P. H. (2005). Caspases: Pharmacological manipulation of cell death. J Clin Invest, 115(10), 2665–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letterio J. J., Roberts A. B. (1998). Regulation of immune responses by TGF-β. Ann Rev Immunol, 16(1), 137–161. [DOI] [PubMed] [Google Scholar]
- Lin L., Huang Q.-X., Yang S.-S., Chu J., Wang J.-Z., Tian Q. (2013). Melatonin in Alzheimer's disease. Int J Mol Sci, 14(7), 14575–14593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López A., García J. A., Escames G., Venegas C., Ortiz F., López L. C., Acuña-Castroviejo D. (2009). Melatonin protects the mitochondria from oxidative damage reducing oxygen consumption, membrane potential, and superoxide anion production. J Pineal Res, 46(2), 188–198. [DOI] [PubMed] [Google Scholar]
- Lu J., Miao J., Su T., Liu Y., He R. (2013). Formaldehyde induces hyperphosphorylation and polymerization of Tau protein both in vitro and in vivo. Biochim Biophys Acta Gen Subjects, 1830(8), 4102–4116. [DOI] [PubMed] [Google Scholar]
- Mandelkow E.-M., Mandelkow E. (2012). Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harbor Perspect Med, 2(7), a006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo E. S., Shin R.-W., Billingsley M. L., Van deVoorde A., O'Connor M., Trojanowski J. Q., Lee V. M. (1994). Biopsy-derived adult human brain tau is phosphorylated at many of the same sites as Alzheimer's disease paired helical filament tau. Neuron, 13(4), 989–1002. [DOI] [PubMed] [Google Scholar]
- Means J. C., Lopez A. A., Koulen P. (2020). Resveratrol protects optic nerve head astrocytes from oxidative stress-induced cell death by preventing caspase-3 activation, tau dephosphorylation at Ser 422 and formation of misfolded protein aggregates. Cell Mol Neurobiol, 40, 911–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirbaha H., Chen D., Morozova O. A., Ruff K. M., Sharma A., Liu X., Pappu R. V., Colby D. W., Mirzaei H., Joachimiak L. A. (2018). Inert and seed-competent tau monomers suggest structural origins of aggregation. Elife, 7, e36584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motilva V., García-Mauriño S., Talero E., Illanes M. (2011). New paradigms in chronic intestinal inflammation and colon cancer: Role of melatonin. J Pineal Res, 51(1), 44–60. [DOI] [PubMed] [Google Scholar]
- Murphy K. E., Park J. J. (2017). Can co-activation of Nrf2 and neurotrophic signaling pathway slow Alzheimer's disease? Int J Mol Sci, 18(6), 1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi G., Kumar A., Sharma S. S. (2011). Melatonin modulates neuroinflammation and oxidative stress in experimental diabetic neuropathy: Effects on NF‐κB and Nrf2 cascades. J Pineal Res 50(2), 124–131. [DOI] [PubMed] [Google Scholar]
- Noble W., Planel E., Zehr C., Olm V., Meyerson J., Suleman F., Gaynor K., Wang L., LaFrancois J., Feinstein B. (2005). Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc Natl Acad Sci, 102(19), 6990–6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn J., Kulhanek D., Nowlin J., Jones R., Praticà D., Rokach J., Stackman R. (2005). Chronic melatonin therapy fails to alter amyloid burden or oxidative damage in old Tg2576 mice: implications for clinical trials. Brain Res, 1037(1-2), 209–213. [DOI] [PubMed] [Google Scholar]
- Reiter R. J., Acuña-Castroviejo D., Tan D.-X., Burkhardt S. (2001). Free radical-mediated molecular damage. Ann New York Acad Sci, 939(1), 200–215. [PubMed] [Google Scholar]
- Reiter R. J., Mayo J. C., Tan D.-X., Sainz R. M., Alatorre-Jimenez M., Qin L. (2016). Melatonin as an antioxidant: under promises but over delivers. J Pineal Res, 61(3), 253–278. [DOI] [PubMed] [Google Scholar]
- Rio D. C., Ares M., Hannon G. J., Nilsen T. W. (2010). Purification of RNA using TRIzol (TRI reagent). Cold Spring Harbor Protocols, 2010(6): pdb.prot5439. [DOI] [PubMed] [Google Scholar]
- Rosales-Corral S., Tan D.-X., Reiter R. J., Valdivia-Velázquez M., Martínez-Barboza G., Pablo Acosta-Martínez J., Ortiz G. G. (2003). Orally administered melatonin reduces oxidative stress and proinflammatory cytokines induced by amyloid-β peptide in rat brain: A comparative, in vivo study versus vitamin C and E. J Pineal Res, 35(2), 80–84. [DOI] [PubMed] [Google Scholar]
- Schetters S. T., Gomez-Nicola D., Garcia-Vallejo J. J., Van Kooyk Y. (2018). Neuroinflammation: Microglia and T cells get ready to tango. Front Immunol, 8, 1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A., Abiega O., Shahraz A., Neumann H. (2013). Janus-faced microglia: beneficial and detrimental consequences of microglial phagocytosis. Front Cell Neurosci, 7, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonawane S. K., Chinnathambi S. (2018). Prion-like propagation of post-translationally modified tau in Alzheimer’s disease: A hypothesis. J Mol Neurosci, 65(4), 480–490. [DOI] [PubMed] [Google Scholar]
- Sun L.-H., Ban T., Liu C.-D., Chen Q.-X., Wang X., Yan M.-L., Hu X.-L., Su X.-L., Bao Y.-N., Sun L.-L., Zhao L.-J., Pei S.-C., Jiang X.-M., Zong D.-K., Ai J. (2015). Activation of Cdk5/p25 and tau phosphorylation following chronic brain hypoperfusion in rats involves microRNA-195 down-regulation. J Neurochem, 134(6), 1139–1151. [DOI] [PubMed] [Google Scholar]
- Suuronen T., Kolehmainen P., Salminen A. (2000). Protective effect of L-deprenyl against apoptosis induced by okadaic acid in cultured neuronal cells. Biochem Pharm, 59(12), 1589–1595. [DOI] [PubMed] [Google Scholar]
- Tremblay M.-È., Stevens B., Sierra A., Wake H., Bessis A., Nimmerjahn A. (2011). The role of microglia in the healthy brain. J Neurosci, 31(45), 16064–16069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi D. N., Jena G. B. (2010). Effect of melatonin on the expression of Nrf2 and NF-kappaB during cyclophosphamide-induced urinary bladder injury in rat. J Pineal Res, 48(4), 324–331. [DOI] [PubMed] [Google Scholar]
- Wajant H., Pfizenmaier K., Scheurich P. (2003). Tumor necrosis factor signaling. Cell Death Diff, 10(1), 45. [DOI] [PubMed] [Google Scholar]
- Walker L. J., Summers D. W., Sasaki Y., Brace E. J., Milbrandt J., DiAntonio A. (2017). MAPK signaling promotes axonal degeneration by speeding the turnover of the axonal maintenance factor NMNAT2. Elife, 6: e22540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D.-L., Ling Z.-Q., Cao F.-Y., Zhu L.-Q., Wang J.-Z. (2004). Melatonin attenuates isoproterenol-induced protein kinase A overactivation and tau hyperphosphorylation in rat brain. J Pineal Res, 37(1), 11–16. [DOI] [PubMed] [Google Scholar]
- Wang X. (2009). The antiapoptotic activity of melatonin in neurodegenerative diseases. CNS Neurosci Therap, 15(4), 345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z., Agarwal-Mawal A., Paudel H. K. (2004). 14-3-3 binds to and mediates phosphorylation of microtubule-associated tau protein by Ser9-phosphorylated glycogen synthase kinase 3β in the brain. J Biol Chem, 279(25), 26105–26114. [DOI] [PubMed] [Google Scholar]
- Zhang H.-M., Zhang Y. (2014). Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J Pineal Res, 57(2), 131–146. [DOI] [PubMed] [Google Scholar]
- Zhou J.-N., Liu R.-Y., Kamphorst W., Hofman M. A., Swaab D. F. (2003). Early neuropathological Alzheimer's changes in aged individuals are accompanied by decreased cerebrospinal fluid melatonin levels. J Pineal Res, 35(2), 125–130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-asn-10.1177_1759091420981204 for Melatonin Reduces GSK3β-Mediated Tau Phosphorylation, Enhances Nrf2 Nuclear Translocation and Anti-Inflammation by Rashmi Das, Abhishek Ankur Balmik and Subashchandrabose Chinnathambi in ASN Neuro