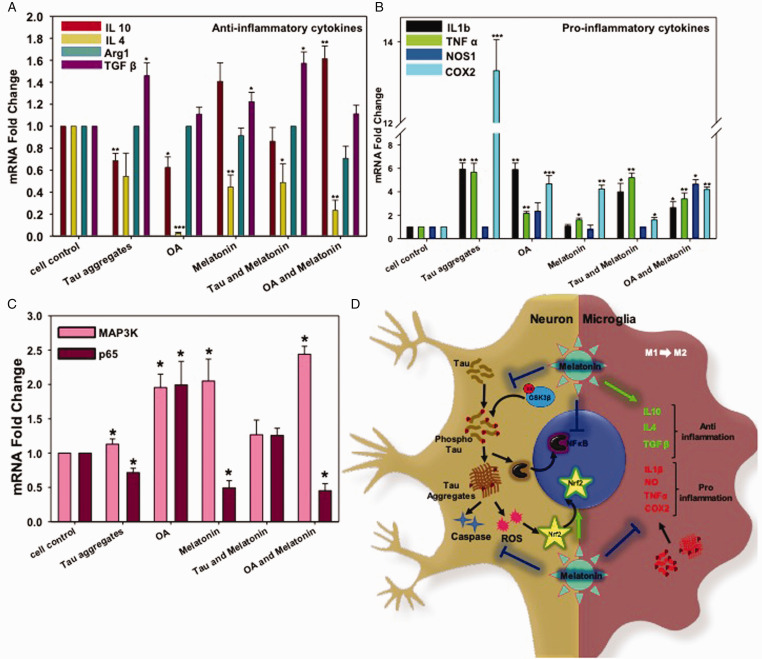
Figure 5.
Real-Time Expression of Pro- and Anti-Inflammatory Cytokines in N9 Cells. A: Melatonin-induced the level of the anti-inflammatory cytokine (TGFβ and IL10) alone and together with either Tau aggregates or OA. B: OA and Tau aggregates induced pro-inflammatory cytokines (IL 1β, TNFα, COX2) excessively, but Melatonin rescued the inflammatory damage by reducing the expression of COX2. IL1β expression level was also brought down by Melatonin treatment upon Tau and OA exposure; however, TNFα mRNA level remains unchanged. C: the mRNA expression level of p65 (NFκB) increased upon Tau and OA treatment, whereas; Melatonin reduced the P65 level compared to untreated group. The MAP3K expression level was increased by 2.5 times upon Melatonin treatment, which is related to neuronal survival. Values were given as mean + SEM. * corresponds to test groups compared with untreated control (*p < 0.05; **p <0.01, ***p<0.001). D: Activation of cellular kinase leads to Tau phosphorylation and aggregates accumulation, which in turn activates ROS production and Caspase-apoptotic signal in neurons. Aggregated Tau can be released through axons, which are eventually uptaken by microglia and returned into the ‘Activation' state. Activated microglia enlarges its extension and produce excess pro-inflammatory cytokines. But Melatonin treatment reduces the activated state of microglia and allows to secrete more anti-inflammatory cytokines, which can manage the burden of aggregates by increased phagocytic activity. Melatonin reduces NFκB activation in inflammation, but MAP3K activation and transition towards the M2 phenotype of microglia lead to axonal repair and survival.