Abstract
Aims
Little is known about patient characteristics, outcomes, and the effect of treatment in relation to duration of heart failure (HF). We have investigated these questions in PARADIGM‐HF. The aim of the study was to compare patient characteristics, outcomes, and the effect of sacubitril/valsartan, compared with enalapril, in relation to time from HF diagnosis in PARADIGM‐HF.
Methods and results
HF duration was categorized as 0–1, >1–2, >2–5, and >5 years. Outcomes were adjusted for prognostic variables, including N‐terminal pro‐brain natriuretic peptide (NT‐proBNP). The primary endpoint was the composite of HF hospitalization or cardiovascular death. The number of patients in each group was as follows: 0–1 year, 2523 (30%); >1–2 years, 1178 (14%); >2–5 years, 2054 (24.5%); and >5 years, 2644 (31.5%). Patients with longer‐duration HF were older, more often male, and had worse New York Heart Association class and quality of life, more co‐morbidity, and higher troponin‐T but similar NT‐proBNP levels. The primary outcome rate (per 100 person‐years) increased with HF duration: 0–1 year, 8.4 [95% confidence interval (CI) 7.6–9.2]; >1–2 years, 11.2 (10.0–12.7); >2–5 years, 13.4 (12.4–14.6); and >5 years, 14.2 (13.2–15.2); P < 0.001. The hazard ratio was 1.26 (95% CI 1.07–1.48), 1.52 (1.33–1.74), and 1.53 (1.33–1.75), respectively, for >1–2, >2–5, and >5 years, compared with 0–1 year. The benefit of sacubitril/valsartan was consistent across HF duration for all outcomes, with the primary endpoint hazard ratio 0.80 (95% CI 0.67–0.97) for 0–1 year and 0.73 (0.63–0.84) in the >5 year group. For the primary outcome, the number needed to treat for >5 years was 18, compared with 29 for 0–1 year.
Conclusions
Patients with longer‐duration HF had more co‐morbidity, worse quality of life, and higher rates of HF hospitalization and death. The benefit of a neprilysin inhibitor was consistent, irrespective of HF duration. Switching to sacubitril/valsartan had substantial benefits, even in patients with long‐standing HF.
Keywords: Heart failure, Duration, Sacubitril/valsartan, Outcomes
Introduction
Large‐scale clinical trials in chronic ambulatory heart failure (HF) usually enrol patients diagnosed at least 1 month previously but rarely report anything further about duration of HF prior to enrolment. Few have reported any data on duration of HF at the time of inclusion, the relation between HF duration and patient characteristics, or whether outcomes vary according to time from diagnosis of HF. 1 , 2 Likewise, little is known about whether duration of HF and chronicity of neurohumoral activation influences the effect of therapy. Potentially, increasing age and co‐morbidity might reduce the benefit of treatment in patients with HF of longer duration. We have investigated these questions further in the Prospective Comparison of ARNI with an ACE‐Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure trial (PARADIGM‐HF), the largest trial to date in patients with HF with reduced ejection fraction (HFrEF).
Our aims were to compare patient characteristics and treatment, co‐morbidities, and functional status according to HF duration, as well as outcomes in relation to time from diagnosis of HF. In addition, we examined the effect of sacubitril/valsartan, compared with enalapril, according to duration of HF prior to enrolment.
Methods
The design, baseline characteristics, and results or PARADIGM‐HF are published. 3 , 4 , 5
Study patients
Patients were eligible if they were ≥18 years, New York Heart Association (NYHA) class II–IV, left ventricular ejection fraction (LVEF) ≤ 35%, elevated natriuretic peptide levels, taking a stable dose of an angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker equivalent to enalapril 10 mg daily for at least 4 weeks, taking a stable dose of beta‐blocker for at least 4 weeks (unless contraindicated or not tolerated), and a mineralocorticoid receptor antagonist, if indicated.
The key exclusion criteria included symptomatic hypotension or systolic blood pressure (SBP) < 95 mmHg, estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2, and potassium > 5.4 mmol/L.
Categorization of heart failure duration
Time from diagnosis was collected in the case report form in the following categories: 0–3 months, >3–6 months, >6–12 months, >1–2 years, >2–5 years, and >5 years. To ensure adequate numbers for analysis in each category, the first three groups were combined, that is, 0–12 months, although all predefined categories were used in the threshold analysis (see below). 6
Trial outcomes
We analysed the primary outcome (a composite of hospitalization for HF or death from cardiovascular causes), the components of the primary outcome, and all‐cause mortality. We also reported recurrent hospitalizations for HF and the two major modes of cardiovascular death, that is, sudden death and death from progressive pump failure. We determined the proportion of patients experiencing a 5 or greater point reduction or improvement from baseline to 8 months in the Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ‐CSS), as this is considered to be a clinically significant difference in health‐related quality of life. 7 , 8
Biomarker measurements
In addition to N‐terminal pro‐brain natriuretic peptide (NT‐proBNP), high‐sensitivity troponin‐T (hsTnT), growth differentiation factor‐15 (GDF‐15), soluble suppression of tumorigenicity‐2 (sST2), tissue inhibitor of matrix metalloproteinase (TIMP)‐1, matrix metalloproteinase (MMP)‐2, MMP‐9, galectin‐3 (Gal‐3), and kidney injury molecule‐1 (KIM‐1) were measured, as previously described. 9 , 10 , 11 , 12
Statistical analysis
Baseline characteristics are reported as means ± standard deviations or medians and inter‐quartile ranges (Q1–Q3) for continuous variables, and frequencies with percentages for categorical variables. A Wilcoxon‐type test for trend (non‐parametric test for trend for categorical variables and a test for trend by means of variance weighted least square regression for continuous variables) was used to compare baseline characteristics between groups. 13
Cox proportional hazard models were used to estimate hazard ratios (HRs) with 95% confidence intervals (CIs) for the time‐to‐event endpoints, and logistic regression analysis was used to determine odds ratios (ORs) for the endpoint of ≥5 points fall or rise in KCCQ‐CSS at 8 months. Along with crude HRs, we report adjusted HRs from models including age, sex, race, previous HF hospitalization, heart rate, SBP, body mass index, NYHA class, LVEF, eGFR, history of myocardial infarction (MI), history of atrial fibrillation, diabetes, NT‐proBNP, and baseline KCCQ‐CSS. These are variables known to be predictors of risk in patients with HF. 14 , 15 ORs were adjusted for all variables listed above, except previous HF hospitalization. All models were adjusted for randomized treatment arm and region.
Recurrent hospitalizations for HF were analysed using the Lin, Wei, Yang, and Ying (LWYY) model. 16 We reported both the crude rate ratio (RR) and RRs adjusted for all variables mentioned above.
The change in mean KCCQ‐CSS at 8 months from baseline was assessed using a repeated‐measures mixed effects model with baseline KCCQ values, region, treatment arm, study visit, and the interaction between study visit and HF duration study group included in the model. The treatment difference of change in mean KCCQ‐CSS at 8 months was assessed using a similar model, but with interaction between study visit and treatment arm used in the model. Interaction between duration of HF and treatment was tested for using the Wald method.
In the analyses of treatment effect, we used Cox proportional hazard models to estimate overall HRs (with 95% CIs), along with HRs according to HF duration for each time‐to‐event endpoint. We used the LWYY model to estimate treatment effect on recurrent HF hospitalizations, and it is shown as RRs. As before, we used logistic regression analysis for the endpoints of ≥5 points fall or rise in KCCQ‐CSS at 8 months, which is reported as ORs. Along with treatment arm and region, this model was adjusted for baseline KCCQ‐CSS. The primary variable of interest was the interaction P‐value for randomized treatment × HF duration. We also performed a threshold analysis, where the HR for the effect of sacubitril/valsartan, compared with enalapril, on the primary endpoint was calculated for each threshold value for HF duration (0.25 to >5 years), using a Cox model adjusted for prognostic variables mentioned earlier. For each threshold value, the model was applied to data for patients with HF duration of at least the threshold value.
A two‐tailed P‐value of <0.05 was considered significant. Statistical analyses were conducted using STATA version 16.0 (Stata Corp., College Station, Texas, USA).
Results
Among the 8399 patients in PARADIGM‐HF, the number in each time‐from‐diagnosis category was as follows: 0–1 year, 2523 (30%); >1–2 years, 1178 (14%); >2–5 years, 2054 (24.5%); and >5 years, 2644 (31.5%).
Baseline characteristics
Most baseline characteristics differed in relation to time since diagnosis of HF (Table 1 ). Some of the largest differences were in age (mean 66.5 years in the HF > 5 year group vs. 61.0 years in the 0–1 year group), aetiology (64.8% ischaemic vs. 52.7%, respectively), and prevalence of co‐morbidities, all of which were substantially more common in patients with longer‐duration HF, with the exception of anaemia where the reverse was true (Table 1 ). The proportion of patients with multiple co‐morbidities also increased with increasing duration of HF (Figure 1 ).
Table 1.
Baseline characteristics according to duration of heart failure
| Characteristic | HF 0–1 year (n = 2523) | HF > 1–2 years (n = 1178) | HF > 2–5 years (n = 2054) | HF > 5 years (n = 2644) | P‐value for trend (P trend) |
|---|---|---|---|---|---|
| Age, years | 61.0 ± 12.1 | 62.4 ± 11.8 | 64.5 ± 10.9 | 66.5 ± 10.1 | <0.001 |
| Age ≥70 years, no. (%) | 651 (25.8) | 344 (29.2) | 713 (34.7) | 1090 (41.2) | <0.001 |
| Female sex, no. (%) | 622 (24.7) | 253 (21.5) | 422 (20.5) | 535 (20.2) | <0.001 |
| Race or ethnic group, no. (%) | <0.001 | ||||
| White | 1353 (53.6) | 711 (60.4) | 1403 (68.3) | 2077 (78.6) | |
| Black | 127 (5.0) | 74 (6.3) | 100 (4.9) | 127 (4.8) | |
| Asian | 718 (28.5) | 256 (21.7) | 312 (15.2) | 223 (8.4) | |
| Other | 325 (12.9) | 137 (11.6) | 239 (11.6) | 217 (8.2) | |
| Region, no. (%) | <0.001 | ||||
| North America | 110 (4.4) | 53 (4.5) | 119 (5.8) | 320 (12.1) | |
| Latin America | 475 (18.8) | 209 (17.7) | 366 (17.8) | 383 (14.5) | |
| Western Europe and other | 509 (20.2) | 250 (21.2) | 474 (23.1) | 818 (30.9) | |
| Central Europe | 715 (28.3) | 414 (35.1) | 793 (38.6) | 904 (34.2) | |
| Asia Pacific | 714 (28.3) | 252 (21.4) | 302 (14.7) | 219 (8.3) | |
| Systolic BP, mmHg | 121 ± 15 | 122 ± 16 | 122 ± 15 | 120 ± 15 | 0.014 |
| Heart rate, b.p.m. | 73 ± 12 | 73 ± 12 | 73 ± 12 | 71 ± 12 | <0.001 |
| BMI, kg/m2 | 27.3 ± 5.5 | 27.7 ± 5.4 | 28.5 ± 5.6 | 29.0 ± 5.4 | <0.001 |
| BMI classification | <0.001 | ||||
| Obesity (BMI ≥ 30) | 690 (27.4) | 343 (29.2) | 685 (33.4) | 1001 (37.9) | |
| Overweight (BMI 25–29.9) | 929 (36.9) | 452 (38.4) | 818 (39.8) | 1050 (39.8) | |
| Normal weight (BMI 18.5–24.9) | 814 (32.3) | 352 (29.9) | 526 (25.6) | 576 (21.8) | |
| Underweight (BMI < 18.5) | 87 (3.5) | 29 (2.5) | 24 (1.2) | 13 (0.5) | |
| Haemoglobin, g/L | 137.7 ± 16.2 | 138.8 ± 16.4 | 140.4 ± 15.8 | 140.4 ± 15.6 | <0.001 |
| Serum creatinine, mg/dL | 1.06 ± 0.3 | 1.10 ± 0.3 | 1.14 ± 0.3 | 1.19 ± 0.3 | <0.001 |
| eGFR, mL/min/1.73 m2 | 72.6 ± 21.8 | 69.8 ± 20.1 | 66.7 ± 18.7 | 62.9 ± 18.2 | <0.001 |
| Clinical HF features | |||||
| Ischaemic cardiomyopathy, no. (%) | 1329 (52.7) | 699 (59.3) | 1295 (63.1) | 1713 (64.8) | <0.001 |
| LVEF, % | 29.3 ± 6.0 | 29.7 ± 6.3 | 29.7 ± 6.3 | 29.3 ± 6.3 | 0.438 |
| Median BNP (IQR), pg/mL | 242 (139–468) | 268 (159–502) | 261 (161–483) | 247 (157–441) | 0.326 |
| Median NT‐proBNP (IQR), pg/mL | 1550 (845–3183) | 1838 (1008–3521) | 1648 (889–3368) | 1570 (888–3016) | 0.948 |
| Median NT‐proBNP (IQR), pg/mL if AF on ECG | 1992 (1115–3700) | 2249 (1220–4038) | 1919 (1174–3908) | 2015 (1209–3833) | 0.754 |
| Median NT‐proBNP (IQR), pg/mL if no AF on ECG | 1450 (804–3021) | 1765 (961–3360) | 1512 (831–3137) | 1428 (790–2798) | 0.255 |
| NYHA class, no. (%) | <0.001 | ||||
| I | 151 (6.0) | 56 (4.8) | 84 (4.1) | 98 (3.7) | |
| II | 1924 (76.3) | 819 (69.5) | 1376 (67.0) | 1800 (68.1) | |
| III | 435 (17.2) | 281 (23.9) | 576 (28.0) | 726 (27.5) | |
| IV | 10 (0.4) | 16 (1.4) | 15 (0.7) | 19 (0.7) | |
| Missing data | 3 (0.1) | 6 (0.5) | 3 (0.2) | 1 (0.0) | |
| KCCQ‐CSS (baseline) | 79.3 ± 18.2 | 75.6 ± 19.4 | 74.8 ± 19.3 | 74.0 ± 19.9 | <0.001 |
| Symptoms and signs, no. (%) | |||||
| Effort dyspnoea | 2115 (84.0) | 1000 (85.3) | 1798 (87.7) | 2294 (86.8) | 0.001 |
| Rest dyspnoea | 65 (2.6) | 31 (2.6) | 106 (5.2) | 107 (4.0) | <0.001 |
| Fatigue | 1234 (49.0) | 603 (51.5) | 1118 (54.5) | 1388 (52.5) | 0.003 |
| Orthopnoea | 182 (7.2) | 89 (7.6) | 139 (6.8) | 198 (7.5) | 0.903 |
| Paroxysmal nocturnal dyspnoea | 100 (4.0) | 57 (4.9) | 125 (6.1) | 117 (4.4) | 0.210 |
| Rales | 169 (6.8) | 96 (8.2) | 196 (9.6) | 202 (7.6) | 0.091 |
| Oedema | 432 (17.2) | 241 (20.6) | 450 (21.9) | 625 (23.6) | <0.001 |
| Jugular venous distention | 238 (9.5) | 120 (10.2) | 209 (10.2) | 251 (9.5) | 0.932 |
| 3rd heart sound | 271 (10.8) | 121 (10.3) | 198 (9.7) | 206 (7.8) | <0.001 |
| Median biomarkers (IQR) | |||||
| Gal‐3, ng/mL | 16.03 (13.16–20.15) | 17.41 (14.64–20.81) | 16.69 (13.41–21.21) | 17.48 (14.32–21.97) | 0.001 |
| GDF‐15, ng/L | 1473 (1005–2126) | 1828 (1284–2552) | 1554 (1100–2184) | 1772 (1281–2644) | <0.001 |
| KIM‐1, pg/mL | 128 (84–180) | 146 (104–221) | 125 (82–192) | 128 (88–192) | 0.629 |
| MMP‐2, ng/mL | 132.64 (114.50–155.69) | 137.64 (115.52–160.86) | 133.03 (114.53–153.67) | 136.03 (118.59–156.69) | 0.350 |
| MMP‐9, ng/mL | 57.57 (38.37–120.61) | 62.26 (40.18–124.52) | 71.73 (39.16–137.39) | 63.67 (38.16–128.26) | 0.599 |
| sST2, ng/mL | 30 (25–40) | 32 (26–42) | 32 (25–41) | 33 (27–42) | 0.003 |
| TIMP1, ng/mL | 121 (101–146) | 127 (107–152) | 124 (106–151) | 126 (105–154) | 0.059 |
| hsTnT, μg/L | 0.014 (0.009–0.022) | 0.0175 (0.012–0.028) | 0.017 (0.01–0.024) | 0.018 (0.012–0.027) | <0.001 |
| Medical history, no. (%) | |||||
| Hypertension | 1646 (65.2) | 825 (70.0) | 1527 (74.3) | 1942 (73.5) | <0.001 |
| Diabetes | 765 (30.3) | 388 (32.9) | 718 (35.0) | 1036 (39.2) | <0.001 |
| Atrial fibrillation (history) | 696 (27.6) | 410 (34.8) | 804 (39.1) | 1181 (44.7) | <0.001 |
| Atrial fibrillation (ECG) | 481 (19.1) | 271 (23.0) | 559 (27.2) | 725 (27.4) | <0.001 |
| Prior HF hospitalization | 1539 (61.0) | 740 (62.8) | 1285 (62.6) | 1710 (64.7) | 0.009 |
| Coronary heart disease | 1121 (44.4) | 609 (51.7) | 1199 (58.4) | 1656 (62.6) | <0.001 |
| MI | 845 (33.5) | 478 (40.6) | 940 (45.8) | 1371 (51.9) | <0.001 |
| Stroke | 149 (5.9) | 98 (8.3) | 194 (9.4) | 284 (10.7) | <0.001 |
| PAD | 98 (3.9) | 54 (4.6) | 125 (6.1) | 217 (8.2) | <0.001 |
| COPD | 244 (9.7) | 155 (13.2) | 291 (14.2) | 390 (14.8) | <0.001 |
| CKD (eGFR < 60 mL/min/1.73 m2) | 692 (27.4) | 391 (33.2) | 766 (37.3) | 1212 (45.8) | <0.001 |
| Anaemia a | 573 (23.3) | 262 (22.9) | 373 (18.8) | 484 (19.1) | <0.001 |
| Treatments at randomization, no. (%) | |||||
| Diuretic | 1981 (78.5) | 977 (82.9) | 1622 (79.0) | 2158 (81.6) | 0.040 |
| Digitalis | 751 (29.8) | 356 (30.2) | 617 (30.0) | 815 (30.8) | 0.443 |
| Beta‐blocker | 2327 (92.2) | 1092 (92.7) | 1902 (92.6) | 2490 (94.2) | 0.010 |
| MRA | 1388 (55.0) | 673 (57.1) | 1144 (55.7) | 1466 (55.4) | 0.885 |
| ICD/CRT‐D | 132 (5.2) | 112 (9.5) | 339 (16.5) | 660 (25.0) | <0.001 |
| CRT‐P/CRT‐D | 51 (2.0) | 55 (4.7) | 138 (6.7) | 330 (12.5) | <0.001 |
AF, atrial fibrillation; BMI, body mass index; BNP, B‐type natriuretic peptide; BP, blood pressure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy with pacemaker (P) or defibrillator (D); ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; Gal‐3, galectin‐3; GDF‐15, growth differentiation factor‐15; HF, heart failure; hsTnT, high‐sensitivity troponin‐T; ICD, implantable cardioverter‐defibrillator; IQR, inter‐quartile range; KCCQ‐CSS, Kansas City Cardiomyopathy Questionnaire clinical summary score; KIM‐1, kidney injury molecule‐1; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MMP‐2, matrix metalloproteinase‐2; MMP‐9, matrix metalloproteinase‐9; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; PAD, peripheral arterial disease; sST2, soluble suppression of tumorigenicity‐2; TIMP‐1, tissue inhibitor of matrix metalloproteinase‐1.
Anaemia: haemoglobin < 130 g/L in men and haemoglobin < 120 g/L in women.
Units: millimetres of mercury (mmHg), beats per minute (b.p.m.), kilograms per metre squared (kg/m2), grams per litre (g/L), milligrams per decilitre (mg/dL), picograms per millilitre (pg/mL), nanograms per litre (ng/L), nanograms per millilitre (ng/mL), and micrograms per litre (μg/L).
Figure 1.
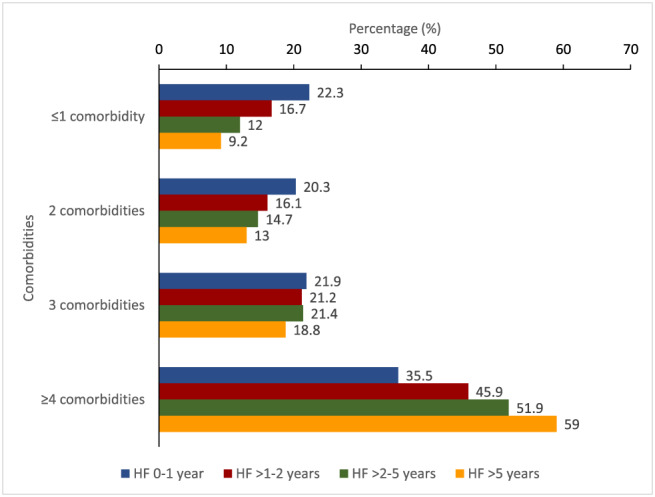
Relationship between duration of heart failure and number of co‐morbidities.
eGFR was considerably lower in patients with longer‐duration HF (62.9 vs. 72.6 mL/min/1.73 m2), and severity of functional limitation was greater (28.2% vs. 17.6% NYHA class III/IV). However, LVEF did not differ notably by duration of HF, and nor did most symptoms or signs, except for dyspnoea (rest or effort) and peripheral oedema (23.6% vs. 17.2%). The difference in the proportion of patients with a history of prior HF hospitalization, in relation to duration of HF, was also modest (64.7% vs. 61.0%). Furthermore, NT‐proBNP level did not differ by duration of HF, although levels of hsTnT (median 0.018 vs. 0.014 μg/L), Gal‐3 (median 17.48 vs. 16.03 ng/mL), GDF‐15 (median 1772 vs. 1473 ng/L), and sST2 (median 33 vs. 30 ng/mL) did.
Treatments at baseline
There were no major differences in pharmacological treatment for HF according to duration of HF. Conversely, there was a five‐fold difference in rates of device therapy in relation to duration of HF. Patients with HF > 5 years were more likely than those in the 0–1 year duration group to have a defibrillating device (25.0% vs. 5.2%) and a cardiac resynchronization device (12.5% vs. 2.0%).
Primary and secondary outcomes in relation to duration of heart failure
Rates of the primary composite outcome of first HF hospitalization or cardiovascular death (per 100 patient‐years) increased with increasing duration of HF: 0–1 year, 8.4 (95% CI 7.6–9.2); >1–2 years, 11.2 (10.0–12.7); >2–5 years, 13.4 (12.4–14.6); and >5 years, 14.2 (13.2–15.2); P < 0.001. The HR, using the HF 0–1 year group as the reference, and adjusting for other prognostic variables, was 1.26 (95% CI 1.07–1.48), 1.52 (1.33–1.74), and 1.53 (1.33–1.75), respectively, for >1–2, >2–5, and >5 years' duration (Table 2 ). Similar trends were seen for the other outcomes, including the components of the primary composite, all‐cause mortality and recurrent hospitalizations for HF. Inspection of the Kaplan–Meier curves (Figure 2 ) suggested the cumulative incidence of cardiovascular (and all‐cause) death was similar in the >2–5 and >5 years' groups for most of the follow‐up period. There appeared to be a clearer gradient between the HF duration groups for the outcomes of first HF hospitalization and recurrent HF hospitalizations.
Table 2.
Event rate (per 100 patient‐years) and risk of study endpoints according to duration of heart failure (HF 0–1 year as reference)
| HF 0–1 year | HF > 1–2 years | HF > 2–5 years | HF > 5 years | P‐Value | |
|---|---|---|---|---|---|
| No. of patients | 2523 | 1178 | 2054 | 2644 | |
| HF hospitalization or cardiovascular death, no. (%) | 442 (17.5) | 270 (22.9) | 569 (27.7) | 750 (28.4) | <0.001 |
| Event rates per 100 patient‐years (95% CI) | 8.4 (7.6–9.2) | 11.2 (10.0–12.7) | 13.4 (12.4–14.6) | 14.2 (13.2–15.2) | |
| Unadjusted HR | 1.00 (ref) | 1.35 (1.16–1.57) | 1.65 (1.46–1.87) | 1.76 (1.56–1.98) | |
| Adjusted a HR | 1.00 (ref) | 1.26 (1.07–1.48) | 1.52 (1.33–1.74) | 1.53 (1.33–1.75) | |
| Cardiovascular death, no. (%) | 295 (11.7) | 160 (13.6) | 346 (16.8) | 450 (17.0) | <0.001 |
| Event rates per 100 patient‐years (95% CI) | 5.3 (4.8–6.0) | 6.2 (5.3–7.2) | 7.5 (6.8–8.4) | 7.7 (7.0–8.5) | |
| Unadjusted HR | 1.00 (ref) | 1.18 (0.97–1.43) | 1.49 (1.27–1.74) | 1.63 (1.40–1.90) | |
| Adjusted a HR | 1.00 (ref) | 1.15 (0.93–1.42) | 1.40 (1.18–1.66) | 1.44 (1.22–1.71) | |
| HF hospitalization, no. (%) | 225 (8.9) | 158 (13.4) | 334 (16.3) | 478 (18.1) | <0.001 |
| Event rates per 100 patient‐years (95% CI) | 4.3 (3.7–4.9) | 6.6 (5.6–7.7) | 7.9 (7.1–8.8) | 9.0 (8.2–9.9) | |
| Unadjusted HR | 1.00 (ref) | 1.54 (1.26–1.89) | 1.86 (1.57–2.21) | 2.05 (1.74–2.42) | |
| Adjusted a HR | 1.00 (ref) | 1.33 (1.07–1.66) | 1.63 (1.36–1.96) | 1.66 (1.39–1.98) | |
| All‐cause mortality (no. of events), no. (%) | 362 (14.3) | 199 (16.9) | 432 (21.0) | 553 (20.9) | <0.001 |
| Event rates per 100 patient‐years (95% CI) | 6.6 (5.9–7.3) | 7.7 (6.7–8.8) | 9.4 (8.5–10.3) | 9.5 (8.7–10.3) | |
| Unadjusted HR | 1.00 (ref) | 1.18 (1.00–1.41) | 1.48 (1.29–1.70) | 1.56 (1.36–1.79) | |
| Adjusted a HR | 1.00 (ref) | 1.15 (0.96–1.39) | 1.37 (1.17–1.60) | 1.35 (1.16–1.58) | |
| Sudden death, no. (%) | 162 (6.4) | 77 (6.5) | 154 (7.5) | 168 (6.4) | 0.826 |
| ‐Event rates per 100 patient‐years (95% CI) | 2.9 (2.5–3.4) | 2.9 (2.3–3.6) | 3.3 (2.8–3.9) | 2.8 (2.4–3.3) | |
| Unadjusted HR | 1.00 (ref) | 1.05 (0.80–1.37) | 1.26 (1.01–1.57) | 1.19 (0.95–1.49) | |
| Adjusted a HR | 1.00 (ref) | 1.09 (0.81–1.46) | 1.23 (0.96–1.57) | 1.19 (0.93–1.53) | |
| Pump failure death, no. (%) | 54 (2.1) | 40 (3.4) | 78 (3.8) | 128 (4.8) | <0.001 |
| Event rates per 100 patient‐years (95% CI) | 1.0 (0.7–1.3) | 1.5 (1.1–2.1) | 1.7 (1.4–2.1) | 2.2 (1.8–2.6) | |
| Unadjusted HR | 1.00 (ref) | 1.56 (1.04–2.36) | 1.73 (1.22–2.45) | 2.32 (1.68–3.22) | |
| Adjusted a HR | 1.00 (ref) | 1.57 (1.01–2.46) | 1.67 (1.14–2.45) | 1.95 (1.35–2.83) | |
| Recurrent HF hospitalizations | |||||
| Total events | 339 | 255 | 536 | 800 | <0.001 |
| Event rates per 100 patient‐years (95% CI) | 6.1 (5.5–6.8) | 9.8 (8.7–11.1) | 11.6 (10.7–12.7) | 13.7 (12.8–14.7) | |
| Unadjusted RR b | 1.00 (ref) | 1.61 (1.37–1.90) | 1.92 (1.68–2.21) | 2.20 (1.93–2.51) | |
| Adjusted a RR | 1.00 (ref) | 1.36 (1.14–1.62) | 1.56 (1.34–1.80) | 1.66 (1.43–1.91) | |
| Significant worsening in KCCQ‐CSS (≥5) at 8 months, d no. (%) | 592 (23.7) | 346 (29.4) | 617 (30.3) | 852 (32.5) | <0.001 |
| Unadjusted OR | 1.00 (ref) | 1.35 (1.15–1.59) | 1.32 (1.16–1.52) | 1.46 (1.28–1.65) | |
| Adjusted c OR | 1.00 (ref) | 1.27 (1.08–1.50) | 1.22 (1.06–1.40) | 1.25 (1.09–1.44) | |
| Significant improvement in KCCQ‐CSS (≥5) at 8 months, d no. (%) | 689 (27.5) | 316 (26.9) | 572 (28.1) | 668 (25.5) | 0.20 |
| Unadjusted OR | 1.00 (ref) | 0.79 (0.66–0.93) | 0.76 (0.66–0.88) | 0.62 (0.54–0.70) | |
| Adjusted c OR | 1.00 (ref) | 0.84 (0.70–0.99) | 0.82 (0.71–0.95) | 0.70 (0.61–0.81) | |
| Change in KCCQ‐CSS at 8 months e (±SE) | −1.19 ± 0.44 | −4.31 ± 0.63 | −4.82 ± 0.46 | −5.10 ± 0.41 | <0.001 |
Model adjusted for age, sex, treatment arm, race, region, previous heart failure hospitalization, heart rate, systolic blood pressure, body mass index, New York Heart Association classification, left ventricular ejection fraction, baseline KCCQ clinical summary score, estimated glomerular filtration rate, history of myocardial infarction, history of atrial fibrillation, diabetes, and NT‐proBNP.
RR denotes rate ratios with 95% confidence intervals (CI) within parentheses, assessed using the LWYY model.
Model adjusted as for (a) except previous heart failure hospitalization.
Scores on the Kansas City Cardiomyopathy Questionnaire (KCCQ) range from 0 to 100 (higher scores indicating fewer symptoms).
Change in mean KCCQ‐CSS at 8 months from baseline was assessed using a repeated‐measures mixed effects model with baseline KCCQ values, region, treatment arm, study visit, and the interaction between study visit and HF duration study group included in the model.
Figure 2.
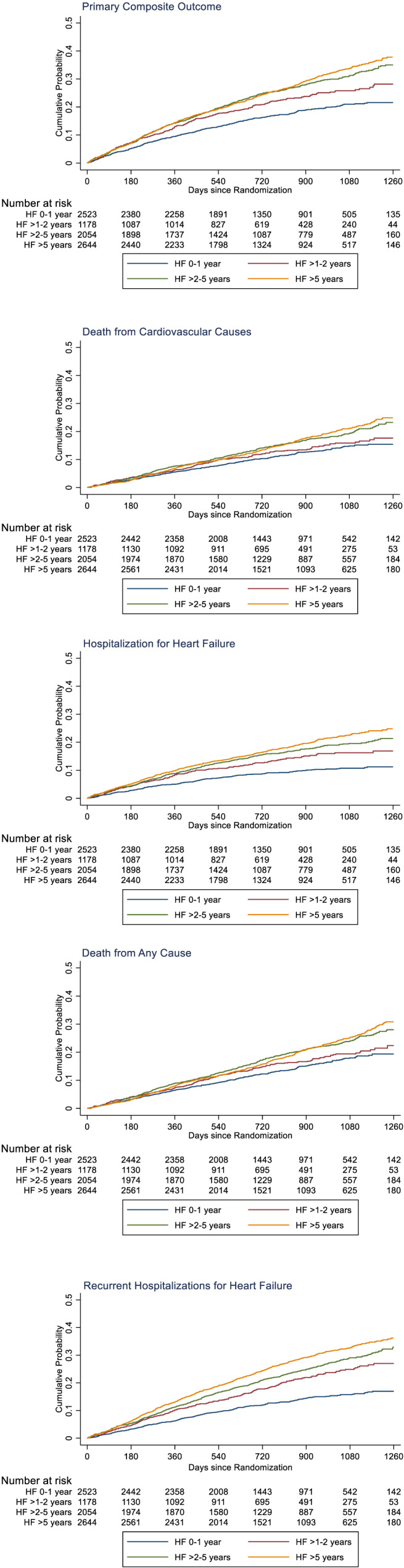
Kaplan–Meier curves for key study outcomes, according to heart failure duration. The panels in this figure show cumulative event curves for primary composite outcome (death from cardiovascular causes or first hospitalization for heart failure), death from cardiovascular causes, first hospitalization for heart failure, death from any cause, and recurrent hospitalizations for heart failure.
There was no significant difference in rates of sudden death regardless of HF duration: 0–1 year, 2.9 (95% CI 2.5–3.4); >1–2 years, 1.5 (1.1–2.1); >2–5 years, 3.3 (2.8–3.9); and >5 years, 2.8 (2.4–3.3) per 100 person‐years, P = 0.826. Conversely, the rate of death from pump failure increased with increasing duration of HF: 0–1 year, 1.0 (0.7–1.3); >1–2 years, 1.5 (1.1–2.1); >2–5 years, 1.7 (1.4–2.1); and >5 years, 2.2 (1.8–2.6) per 100 person‐years, P < 0.001 (Table 2 ).
All groups had a decrease (deterioration) in KCCQ‐CSS between baseline and 8 months. The extent of reduction in KCCQ‐CSS increased with duration of HF. The reduction in mean score from baseline to month 8 was 1.19 points in the 0–1 year group, 4.31 points in the >1–2 year group, 4.82 points in the >2–5 year group, and 5.10 points in the >5 year group (P < 0.001).
Effects of sacubitril/valsartan according to duration of heart failure
The benefit of sacubitril/valsartan was consistent in relation to duration of HF for all outcomes examined (Table 3 ). For example, the HR for the primary endpoint in the trial overall was 0.80 (95% CI 0.73–0.87); in the 0–1 year group, it was 0.80 (0.67–0.97); and in the >5 year group, it was 0.73 (0.63–0.84), interaction P = 0.31. As a result, the absolute benefit was greatest in those with the longest duration of HF; for example, applying the overall 20% relative risk reduction to the event rate in the enalapril group gave an absolute risk reduction of 3.50% and a number needed to treat (NNT) of 29 in patients with HF 0–1 year, compared with an absolute risk reduction of 5.7% and NNT of 18 in patients with HF >5 years (over a median follow‐up of 27 months).
Table 3.
Treatment effect according to duration of heart failure (sacubitril/valsartan vs. enalapril hazard ratio or difference and 95% confidence interval)
| Overall HR (95% CI) or difference | HF 0–1 year HR (95% CI) or difference | HF > 1–2 years HR (95% CI) or difference | HF > 2–5 years HR (95% CI) or difference | HF > 5 years HR (95% CI) or difference | P for interaction | |
|---|---|---|---|---|---|---|
| HF hospitalization or cardiovascular death | 0.80 (0.73–0.87) | 0.80 (0.67–0.97) | 0.95 (0.75–1.21) | 0.83 (0.70–0.98) | 0.73 (0.63–0.84) | 0.3089 |
| Cardiovascular death | 0.80 (0.71–0.89) | 0.74 (0.59–0.94) | 0.83 (0.61–1.14) | 0.95 (0.77–1.17) | 0.73 (0.61–0.88) | 0.3066 |
| HF hospitalization | 0.79 (0.71–0.89) | 0.75 (0.58–0.97) | 1.12 (0.82–1.54) | 0.83 (0.67–1.04) | 0.71 (0.59–0.85) | 0.0837 |
| All‐cause mortality | 0.84 (0.76–0.93) | 0.78 (0.63–0.96) | 0.88 (0.67–1.17) | 1.01 (0.83–1.22) | 0.77 (0.65–0.91) | 0.1754 |
| Sudden death | 0.80 (0.68–0.94) | 0.78 (0.57–1.06) | 0.70 (0.45–1.11) | 0.85 (0.62–1.17) | 0.83 (0.61–1.13) | 0.9173 |
| Pump failure death | 0.85 (0.68–1.07) | 0.83 (0.49–1.42) | 1.08 (0.58–2.02) | 1.05 (0.67–1.64) | 0.70 (0.49–0.99) | 0.4407 |
| Recurrent HF hospitalizations a | 0.78 (0.68–0.90) | 0.64 (0.47–0.88) | 1.15 (0.80–1.65) | 0.93 (0.71–1.20) | 0.67 (0.54–0.84) | 0.0252 |
| Significant worsening in KCCQ‐CSS b (≥5) at 8 months c | 0.82 (0.74–0.90) | 0.81 (0.67–0.98) | 1.03 (0.79–1.34) | 0.79 (0.65–0.95) | 0.78 (0.66–0.92) | 0.2867 |
| Significant improvement in KCCQ‐CSS b (≥5) at 8 months c | 1.09 (0.98–1.21) | 1.11 (0.91–1.35) | 1.03 (0.77–1.37) | 1.06 (0.86–1.31) | 1.14 (0.94–1.37) | 0.9068 |
| Change in KCCQ‐CSS at 8 months d | 1.64 (0.73–2.56) | 0.64 (−1.00–2.29) | 1.01 (−1.52–3.54) | 1.98 (0.05–3.90) | 2.39 (0.84–3.95) | 0.2950 |
Effect of sacubitril/valsartan on recurrent HF hospitalizations was assessed using the LWYY model and is shown as rate ratios (RRs).
Scores on the Kansas City Cardiomyopathy Questionnaire (KCCQ) range from 0 to 100 (higher scores indicating fewer symptoms).
Effect of sacubitril/valsartan on improvement or worsening KCCQ clinical summary score (≥5) at 8 months was estimated by logistic regression and is shown as odds ratios (ORs).
The treatment difference of change in mean KCCQ‐CSS at 8 months from baseline was assessed using a repeated‐measures mixed effects model with baseline KCCQ values, region, treatment arm, study visit, and the interaction between study visit and treatment arm used in the model.
In the threshold analysis, the benefit of sacubitril/valsartan, compared with enalapril, on the primary endpoint was consistent for each threshold value for HF duration (0.25 to >5 years) (Figure 3 ). The HR for the primary endpoint (adjusted for prognostic variables) was 0.83 (95% CI 0.75–0.91) for patients with HF duration > 0.25 years, 0.82 (0.74–0.90) if HF duration > 0.5 years, 0.81 (0.73–0.90) if HF duration > 1 year, 0.78 (0.70–0.88) if HF duration > 2 years, and 0.76 (0.66–0.89) if HF duration > 5 years.
Figure 3.
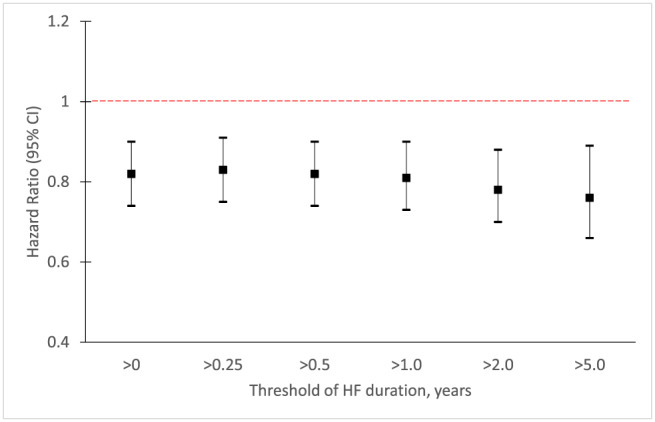
Treatment effect of sacubitril/valsartan on the primary composite outcome (cardiovascular death or first hospitalization for heart failure) according to threshold duration of heart failure. Treatment effect for the primary composite outcome using Cox model adjusted for prognostic variables as per Table 2 (a), according to threshold duration of heart failure. CI, confidence interval.
Discussion
Two aspects of the current findings are worthy of discussion. First, we provide one of the few descriptions of how patient characteristics and outcomes vary by time from diagnosis in patients with chronic HFrEF. Second, we examined whether the effects of treatment were modified by duration of HF.
There has been much debate about the heterogeneity of patients admitted to hospital with acute HF, with several studies focusing on the importance of duration of HF. In those reports, particular attention was paid to comparison of patients presenting ‘de novo’ and those with acute worsening of chronic HF. 17 , 18 , 19 , 20 , 21 , 22 By contrast, little has been written about heterogeneity related to duration of HF in the ambulatory setting. Indeed, we could find only one other report, which was from the Systolic Heart failure treatment with the If inhibitor ivabradine Trial (SHIFT). 2 As in SHIFT, and in the studies of patients hospitalized with HF mentioned above, we found that patients with longer‐duration HF were older and more often had coronary heart disease. While the former finding is not surprising, the latter is not so intuitive. As patients with concomitant coronary artery disease have a worse prognosis than patients without, a survivor bias in favour of patients with a non‐ischaemic aetiology might be expected. However, recovery of left ventricular systolic function is less common in ischaemic patients, and the preponderance of the latter patients in HFrEF trials probably reflects this alternative bias. Likewise, the smaller proportion of women with longer‐duration HF probably reflects the male predominance of coronary heart disease, especially as women with HFrEF have a better survival, overall, than men. Other differences between individuals with a longer‐duration and shorter‐duration HF are not unexpected in view of the foregoing discussion, for example, worse symptoms (reflecting the progressive nature of HF over time), more chronic obstructive pulmonary disease (a smoking‐related disease, like coronary heart disease), worse renal function and more atrial fibrillation (both strongly age‐related co‐morbidities), and more diabetes and hypertension (both age‐related and associated with coronary heart disease). Indeed, the burden of co‐morbidity overall was much greater in patients with a longer history of HF, likely contributing to worse prognosis of these patients and possibly reducing the tolerability and effectiveness of therapy. This potential for this heterogeneity to influence response to therapy has been highlighted in the acute HF literature.
We also identified some new findings related to duration of HF. We measured a panel of biomarkers, which was not done in SHIFT. Surprisingly, patients with longer‐duration of HF had a similar median NT‐proBNP concentration to patients with shorter‐duration HF, despite their worse overall clinical picture and higher prevalence of atrial fibrillation. Whether this just reflects survivor bias is uncertain. It is notable that in contrast to NT‐proBNP, individuals with longer‐duration HF had higher high‐sensitivity troponin‐T, sST2, Gal‐3, and GDF‐15 levels, consistent with more advanced HF, although the differences between groups were small. This raises the question of whether the adaptive natriuretic peptide response in HF is preserved in the long term. 23 , 24 , 25
Turning to outcomes, we also identified some new findings in relation to duration of HF. First, we examined a patient‐reported outcome, the KCCQ, which has not been done before. Overall, KCCQ‐CSS decreased (deteriorated) from baseline to 8 months in PARADIGM‐HF. The size of decrease increased in a stepwise fashion with duration of HF, exceeding the clinically meaningful threshold of 5 points in those with an HF duration of >5 years (compared with a mean decrease of 1.19 points in patients with a duration of 0–1 year). 8 The proportion of patients with a ≥5‐point decrease in KCCQ‐CSS was significantly larger (and the proportion with a ≥ 5 point increase smaller) in individuals with longer‐duration compared with shorter‐duration HF. Second, we found a graded relationship between duration of HF and morbidity/mortality outcomes, which is consistent with most, but not all, prior acute HF trials and with SHIFT. However, the pattern of augmented risk was different for mortality and hospitalization. There was a clear stepwise increment in risk of hospital admission across each of the pre‐specified time periods (although the gradient was less steep between >2–5 and >5 years than between 0–to 1 and >1–2 years); this was apparent for recurrent as well as first admissions. However, the risk of death appeared to be similar in the 0–1 and >1–2 year periods and in the >2–5 and >5 year periods. Third and perhaps most importantly, the incremental risk associated with a longer duration of HF persisted after extensive multivariable adjustment. This suggests that the excess risk related to duration of HF is not wholly explained by age and co‐morbidity and likely reflects the progressive neurohumoral, myocardial, renal, and other manifestations of HF and their consequences. Therefore, modifiable risk related to disease activity per se persists remains, even in long‐standing HF.
In keeping with this, we found no evidence of diminution of the effect of sacubitril/valsartan with duration of HF. The benefit over enalapril was consistent over the time periods examined for all the outcomes of interest, whether fatal or non‐fatal. The benefit in the patients with HF of the longest duration was notable in that these patients had the highest rate of use of device therapy (five‐fold higher than in the shortest‐duration patients), as well as consistently good pharmacological treatment. Importantly, because the event rate was substantially higher in patients with longer‐duration HF, the similar relative risk reduction translated into a larger absolute risk reduction in those with a HF duration > 5 years—their NNT for the primary outcome was only 18, compared with 29 for patients with HF of 0–1 year duration.
Study limitations
As in any study of this type, there are limitations. The analyses conducted were not pre‐specified. The patients studied were selected for a clinical trial and will differ from those in ordinary practice. Our study has strengths as well. It used a large, contemporary, geographically representative clinical trial dataset, with well‐characterized patients and an extensive range of adjudicated outcomes.
In summary, patients with longer‐duration HF were older, had more co‐morbidity, worse quality of life, and higher rates of hospitalization and death. However, the benefits of sacubitril/valsartan over enalapril were similar irrespective of HF duration, and if anything, greater in longer‐duration HF. While early treatment with a neprilysin inhibitor may be preferable to improve quality of life and outcomes, the current data show that it is not too late to switch to sacubitril/valsartan in individuals with established HF and that substantial benefits may be obtained in these patients.
Conflict of interest
S.E.Y. and P.D. have no conflict of interest to declare. J.J.V.M., A.S.D., S.D.S., J.L.R., K.S., M.R.Z., P.S.J., and M.P. have consulted for or received research support from Novartis, sponsor of the PARADIGM‐HF trial. M.L. and A.R. are employees of Novartis.
Funding
J.J.V.M. is supported by a British Heart Foundation Centre of Research Excellence (grant RE/18/6/34217).
Yeoh, S. E. , Dewan, P. , Desai, A. S. , Solomon, S. D. , Rouleau, J. L. , Lefkowitz, M. , Rizkala, A. , Swedberg, K. , Zile, M. R. , Jhund, P. S. , Packer, M. , and McMurray, J. J. V. (2020) Relationship between duration of heart failure, patient characteristics, outcomes, and effect of therapy in PARADIGM‐HF. ESC Heart Failure, 7: 3355–3364. 10.1002/ehf2.12972.
References
- 1. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure. J Am Coll Cardiol 2014; 63: 1123–1133. [DOI] [PubMed] [Google Scholar]
- 2. Böhm M, Komajda M, Borer JS, Ford I, Maack C, Tavazzi L, Moyne A, Swedberg K, SHIFT Investigators . Duration of chronic heart failure affects outcomes with preserved effects of heart rate reduction with ivabradine: findings from SHIFT. Eur J Heart Fail 2018; 20: 373–381. [DOI] [PubMed] [Google Scholar]
- 3. McMurray JJVV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 4. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau J, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM‐HF Committees and Investigators . Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin‐converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM‐HF). Eur J Heart Fail 2013; 15: 1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz M, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM‐HF Committees Investigators . Baseline characteristics and treatment of patients in prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM‐HF). Eur J Heart Fail 2014; 16: 817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simpson J, Jhund PS, Silva Cardoso J, Martinez F, Mosterd A, Ramires F, Rizkala AR, Senni M, Squire I, Gong J, Lefkowitz MP, Shi VC, Desai AS, Rouleau JL, Swedberg K, Zile MR, McMurray J, Packer M, Solomon SD, PARADIGM‐HF Investigators and Committees . Comparing LCZ696 with enalapril according to baseline risk using the MAGGIC and EMPHASIS‐HF risk scores: an analysis of mortality and morbidity in PARADIGM‐HF. J Am Coll Cardiol 2015; 66: 2059–2071. [DOI] [PubMed] [Google Scholar]
- 7. Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, Rumsfeld JS, Cardiovascular Outcomes Research Consortium . Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J 2005; 150: 707–715. [DOI] [PubMed] [Google Scholar]
- 8. Flynn KE, Lin L, Moe GW, Howlett JG, Fine LJ, Spertus JA, McConnell TR, Piña IL, Weinfurt KP. Relationships between changes in patient‐reported health status and functional capacity in outpatients with heart failure. Am Heart J 2012; 163: 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zile MR, O'Meara E, Claggett B, Prescott MF, Solomon SD, Swedberg K, Packer M, McMurray JJV, Shi V, Lefkowitz M, Rouleau J. Effects of sacubitril/valsartan on biomarkers of extracellular matrix regulation in patients with HFrEF. J Am Coll Cardiol 2019; 73: 795–806. [DOI] [PubMed] [Google Scholar]
- 10. Rørth R, Jhund PS, Kristensen SL, Desai AS, Køber L, Rouleau JL, Solomon SD, Swedberg K, Zile MR, Packer M, McMurray JJV. The prognostic value of troponin T and N‐terminal pro B‐type natriuretic peptide, alone and in combination, in heart failure patients with and without diabetes. Eur J Heart Fail 2019; 21: 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bouabdallaoui N, Claggett B, Zile MR, McMurray JJV, O'Meara E, Packer M, Prescott MF, Swedberg K, Solomon SD, Rouleau JL, PARADIGM‐HF Investigators and Committees . Growth differentiation factor‐15 is not modified by sacubitril/valsartan and is an independent marker of risk in patients with heart failure and reduced ejection fraction: the PARADIGM‐HF trial. Eur J Heart Fail 2018; 20: 1701–1709. [DOI] [PubMed] [Google Scholar]
- 12. O'Meara E, Prescott MF, Claggett B, Rouleau JL, Chiang LM, Solomon SD, Packer M, McMurray JJV, Zile MR. Independent prognostic value of serum soluble ST2 measurements in patients with heart failure and a reduced ejection fraction in the PARADIGM‐HF Trial (Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure). Circ Heart Fail 2018; 11: e004446. [DOI] [PubMed] [Google Scholar]
- 13. Cuzick J. A Wilcoxon‐type test for trend. Stat Med 1985; 4: 87–90. [DOI] [PubMed] [Google Scholar]
- 14. Rahimi K, Bennett D, Conrad N, Williams TM, Basu J, Dwight J, Woodward M, Patel A, McMurray JJ, MacMahon M. Risk prediction in patients with heart failure. J Am Coll Cardiol HF 2014; 2: 440–446. [DOI] [PubMed] [Google Scholar]
- 15. Kristensen SL, Martinez F, Jhund PS, Arango JL, Bĕlohlávek J, Boytsov S, Cabrera W, Gomez E, Hagège AA, Huang J, Kiatchoosakun S, Kim KS, Mendoza I, Senni M, Squire IB, Vinereanu D, Wong RC, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, Packer M, McMurray JJ. Geographic variations in the PARADIGM‐HF heart failure trial. Eur Heart J 2016; 37: 3167–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin DY, Wei LJ, Yang I, Ying Z. Semiparametric regression for the mean and rate functions of recurrent events. J Royal Stat Soc Series B 2000; 62: 711–730. [Google Scholar]
- 17. Greene SJ, Hernandez AF, Dunning A, Ambrosy AP, Armstrong PW, Butler J, Cerbin LP, Coles A, Ezekowitz JA, Metra M, Starling RC, Teerlink JR, Voors AA, O'Connor CM, Mentz RJ. Hospitalization for recently diagnosed versus worsening chronic heart failure: from the ASCEND‐HF Trial. J Am Coll Cardiol 2017; 69: 3029–3039. [DOI] [PubMed] [Google Scholar]
- 18. Pranata R, Tondas AE, Yonas E, Vania R, Yamin M, Chandra A, Siswanto BB. Differences in clinical characteristics and outcome of de novo heart failure compared to acutely decompensated chronic heart failure—systematic review and meta‐analysis. Acta Cardiol 2020; 1–11. 10.1080/00015385.2020.1747178 [DOI] [PubMed] [Google Scholar]
- 19. Younis A, Mulla W, Goldkorn R, Klempfner R, Peled Y, Arad M, Freimark D, Goldenberg I. Differences in mortality of new‐onset (de‐novo) acute heart failure versus acute decompensated chronic heart failure. Am J Cardiol 2019; 124: 554–559. [DOI] [PubMed] [Google Scholar]
- 20. Butt JH, Fosbøl EL, Gerds TA, Andersson C, McMurray JJV, Petrie MC, Gustafsson F, Madelaire C, Kristensen SL, Gislason GH, Torp‐Pedersen C, Køber L, Schou M. Readmission and death in patients admitted with new‐onset versus worsening of chronic heart failure: insights from a nationwide cohort. Eur J Heart Fail 2020. 10.1002/ejhf.1800 [DOI] [PubMed] [Google Scholar]
- 21. Khan MS, Butler J, Greene SJ. The real world of de novo heart failure: the next frontier for heart failure clinical trials? Eur J Heart Fail 2020. 10.1002/ejhf.1844 [DOI] [PubMed] [Google Scholar]
- 22. Choi KH, Lee GY, Choi JO, Jeon ES, Lee HY, Cho HJ, Lee SE, Kim MS, Kim JJ, Hwang KK, Chae SC, Baek SH, Kang SM, Choi DJ, Yoo BS, Kim KH, Park HY, Cho MC, Oh BH. Outcomes of de novo and acute decompensated heart failure patients according to ejection fraction. Heart 2018; 104: 525–532. [DOI] [PubMed] [Google Scholar]
- 23. van den Berg MP, van Gelder IC, van Veldhuisen DJ. Depletion of atrial natriuretic peptide during longstanding atrial fibrillation. Europace 2004; 6: 433–437. [DOI] [PubMed] [Google Scholar]
- 24. Miller WL, Burnett JC Jr, Hartman KA, Henle MP, Burritt MF, Jaffe AS. Lower rather than higher levels of B‐type natriuretic peptides (NT‐pro‐BNP and BNP) predict short‐term mortality in end‐stage heart failure patients treated with nesiritide. Am J Cardiol 2005; 96: 837–841. [DOI] [PubMed] [Google Scholar]
- 25. Sun TW, Wang LX. Low levels of B‐type natriuretic peptide predict poor clinical outcomes in patients with chronic and advanced heart failure. Med Hypotheses 2007; 68: 677–679. [DOI] [PubMed] [Google Scholar]


