Fig. 1.
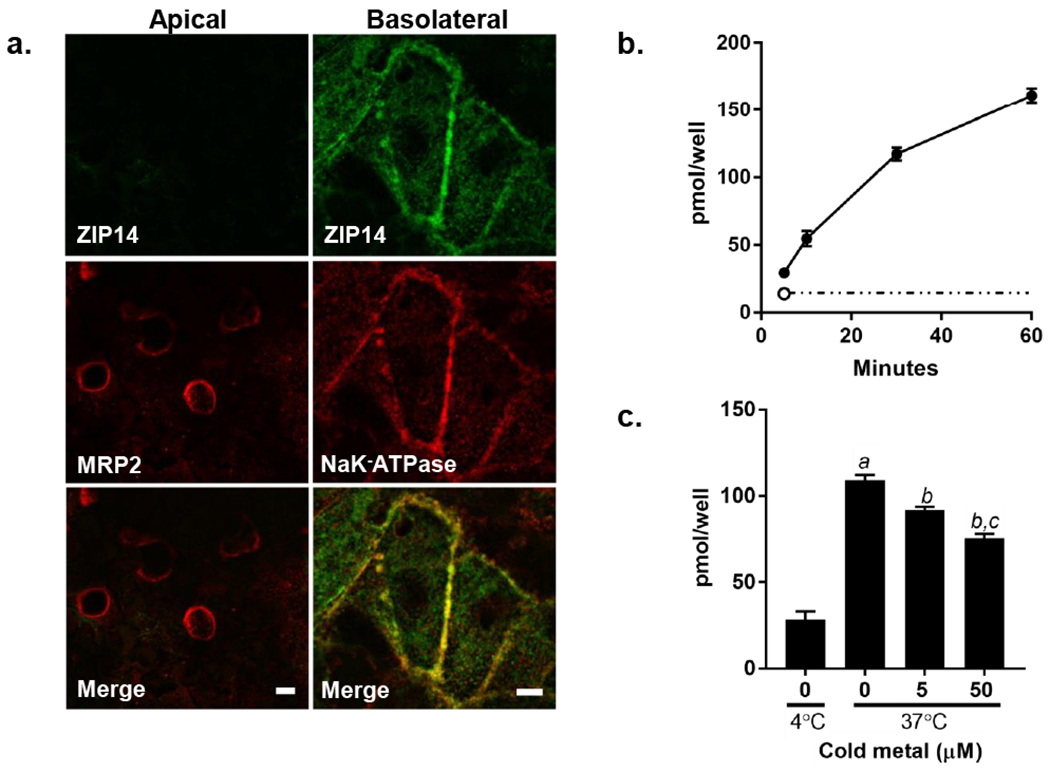
ZIP14 localization and Mn uptake by HepaRG cells. a. ZIP14 colocalizes with the basolateral membrane marker NaK-ATPase but not the apical marker MRP2. HepaRG cells were grown on 0.4um transparent inserts for 12 days in standard culture conditions. Shown are staining for ZIP14 (green) and the apical marker MRP2 (left panel; red) or the basolateral marker NaK-ATPase (right panel; red) using indirect immunofluorescence. Cell were imaged using a Yokagawa CSU-X1 spinning disk confocal system with a Nikon Ti-E inverted microscope using a 60x (MRP2 images) or a 100x (NaK-ATPase images) Plan Apo objective lens with Zyla cMOS camera using 561 and 488 lasers. Bar = 5μm. b. Mn uptake is time and temperature dependent. HepaRG cells were incubated with 1μM 54Mn for 5, 10, 30 and 60 minutes at 37°C (filled circles). Uptake at 4°C is indicated at 5 minutes (open circle) and extrapolated out to 60 minutes as indicated by the dashed line. After incubation for the times indicated, cells were washed, removed from the plate, and cell-associated 54Mn was measured by gamma counting. Values are means ± SEM, n=4 biological samples. c. 54Mn uptake is competed by excess unlabeled Mn. Cells were incubated with 1μM 54Mn in uptake buffer containing either 5 or 50μM cold Mn. After incubation for the times indicated, cells were washed, removed from the plate, and cell-associated 54Mn was measured by gamma counting. Values are means ± SEM, n=4 biological samples; a, significant from 4°C; b, significant from control; c, significant from 5μM Mn. P < 0.05.
